VI. Experimental study of sulfide equilibria and sulfide-forming fluid (Leader Dr. N.S.Gorbachev, Dr. T.P.Dadze)
Gorbachev P.N. and Bezmen N.I. Solubility of sulfur in water-saturated anortite-diopside melts under various redox conditions
The effect of redox conditions on the solubility of sulfur under the pressure of gases of the H-O-S system in melts of the anortite-diopsite composition close to eutectics (An35Ab10Di55, mol.%) was experimentally studied on a high-gas-pressure equipment at a temperature of 1200oC. The powdered sample (150 mg) was molded in a platinum capsule with a diameter of 5 mm and a height of 25 mm. Then sulfur (15-30 mg) and water (50 ml) were added. The fugacity of sulfur was monitored by the Pt-PtS buffer reaction and amounted logfS2 = -0.394. The fugacity of hydrogen was specified outside by argon-hydrogen mixtures in which the molar fraction of hydrogen varied from 0.05 to 0.3. The capsule was sealed and placed in another capsule (diameter 8 mm) that also contained water. This second capsule is needed to restrict diffusion of oxygen and sulfur from the reaction capsule, which is observed at high temperatures. The pressure and composition of the fluid (Table 1) were selected in such a way that the fugacity of water in experiments corresponded to the fugacity of water in the H-O system at 1200oC, 2 kbar, and a specified molar fraction of hydrogen. The duration of experiments was 3 days. The temperature during experiments was monitored with an accuracy of +5oC. The pressure was monitored with an accuracy of +50 bar. The products of experiments were studied by optical methods and on the microprobe.
The results of experiments showed that the solubility of sulfur varies within 0.018 - 0.126 wt.% and is independent of the fugacity of H2S. The highest value of solubility falls on the experiment with the molar fraction of hydrogen of 0.046, and just under these redox conditions, and the melting temperature decreases under the pressure of fluids of the H-O system [1, 2].
Table 1. Physicochemical conditions in experiments on studying the solubility of sulfur
Entry no |
P, bar |
XH2 |
fH2O |
fH2S |
fSO2 |
lgfO2 |
CS % |
1 |
2166 |
0.046 |
1938 |
315 |
0.34 |
-9.30 |
0.126 |
2 |
2347 |
0.080 |
1836 |
683 |
0.06 |
-10.02 |
0.064 |
3 |
2640 |
0.138 |
1632 |
1358 |
0.01 |
-10.72 |
0.034 |
4 |
3211 |
0.248 |
1019 |
3166 |
- |
-11.86 |
0.018 |
References:
#Gorbachev N.S., Nekrasov A.N., Gorbachev P.N. Sulfur solubility in hydrous magmas at high pressures.
Sulfide - silicate liquation of silicate melts plays an important part in the processes of magmatic differentiation and genesis of sulfide Pt-Cu-Ni deposits. Many intrusives with commercial sulfide mineralization incorporate primary magmatic amphibole which is suggestive of high H2O pressure at magma crystallization. In order to understand the conditions of sulfide-silicate liquation of water-bearing magmas and the genesis of the related deposits, we have studied solubility of sulfur in water-bearing (2-4 wt% H2O) magnesial (>12 wt % MgO) silicate melts at P=1-25 kbar, T=1250oC with a quartz-fayalite-magnetite-pyrrhotine O2-S2 buffer.
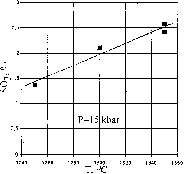
Depending on the T-P-X parameters the solubility of sulfur in water-bearing melts varied from 0.6 to 2.6 wt% SO3 which is an order of magnitude (and even more) higher than that in "dry" melts under the same parameters (figs 1,2). The solubility of sulfur increases by a factor of 1.9, i.e. from 1.37 to 2.58 wt% SO3 with the temperature elevation from 1250 to 1350oC(fig.1). The pressure dependence of solubility exhibits an extremal course with a maximum in the region of 11-15 kbar (fig.2). The dependence of solubility on the melt composition is more intricate. There is a trend to sulfur solubility increase with increasing magnesiality Mg*=MgO/MgO+FeO (and the inverse silicoacidity) of the melt. For ferruginous compositions (11-16 wt% FeO) no positive dependence of sulfur solubility on FeO content was found (fig.3a,b).
Water-bearing magmas, as compared with "dry" ones, possess high potential ore-bearing capacity due to their unique capability of dissolving large amounts of sulfur, chlorine, noble metals (Pt, Au). Due to high solubility, high sulfur concentrations are required for reaching the sulfide saturation of water-bearing melts, therefore the sulfide-silicate liquation, particularly an earlier one, is difficult in such melts. An important factor, contributing to reaching the sulfide saturation of water-bearing magmas, can be an alteration of their chemical composition, i.e., an increase of the sulfur concentration and silicoacidity in the melt, a decrease of its basicity resultant from crust contamination, crystallizational differentiation.
#The work has been sponsored by the RFBR (Grant N 97-05-65925).
75
Fig.1. Sulfur solubility (recalculated for SO3) vs the temperature curve for water-bearing silicate melts
Fig.2. Sulfur solubility (recalculated for SO3) vs the pressure curve for water-bearing silicate melts
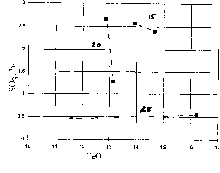 Fig.3.Concentration of SO3 vs FeO (a), SO3 vs Mg* (b) diagram for water-bearing melts. Numbers on the graphs stand for pressure in kbar.
Fig.3.Concentration of SO3 vs FeO (a), SO3 vs Mg* (b) diagram for water-bearing melts. Numbers on the graphs stand for pressure in kbar.
#Dadze T.P., Kashirtseva G.A., Akhmedzhanova G.M. Study of gold solubility in sulphide-bearing solutions at T=300oC.
We have carried out an experimental study of gold solubility in H2S-bearing solutions. Hydrogen sulphide in the system was given by thioacetamide (CH3CSNH2) which made it possible to vary mH2S in a broad concentration range. An analysis of gold was performed on an atom-absorption spectrometer in air-acetylene flame, the wavelength being 242.8 n .
.
The solubility of gold was defined at T=300oC and P of saturated water vapour (pHstart=7.4). Earlier such experiment was conducted at P=300 at. The pH value of the solution ion with added thioacetamide, calculated by Yu.V.Shvarov's "GIBBS" program [2] using the thermodynamic data base UNITHERM [3], at the run parameters changes insignificantly with the increasing concentration of the sulphide sulphur and equals 5.68 at mH2S=0.05 and 5.53 at mH2S=0.5. The results of the investigations and calculations have shown that under the conditions of this particular run two hydrosulphide gold complexes forming via reactions: Au+2H2S(aq) = HAu(HS)2o +1/2 H2(g), and Au+H2S+HS- = Au(HS)2- +1/2 H2(g) are dominant in the solution. The"GIBBS" calculations of the pH dependence of partitioning of gold hydrodsulphide complexes have shown that at pH<4 the dominant forms are HAu(HS)2o and Au(HS)o. Fig.1. illustrates the dependence of partitioning of hydrosulphide gold complexes on pH, calculated from the published data for gold complexes HAu(HS)2o [4] Au(HS)o [5], and Au(HS)2- [6].
Our studies have shown that as the pressure drops from 300 atm to Psat, other conditions being equal, the solubility of gold grows (fig.2). The dependence of the gold solubility on the concentration of sulphide sulphur is expressed by the equations:
lg mAu =-3.27197 + 1.45035.lg mH2S ± 0.17 (P=300 atm.)
lg mAu =-2.65116 + 1.48774.lg mH2S ± 0.004 (Psat.)
Two run series were conducted at T=300oC, P=300 atm and pHstart=2.88 (mHCl=0.0013); the first series without added Na2SO4, the second series with added 0.0469 mole/l Na2SO4. In the system without added Na2SO4 the pH value, calculated at the run parameters, was 5.5 and was, practically, invariant with increasing sulphide sulphur concentration. Also, the addition of sodium sulphate alkalizes noticeably the solution to pH=7 which, possibly, primarily accounts for the fact that gold solubility in the system with added Na2SO4, other conditions being equal, is an order of magnitude higher than in the system without Na2SO4 (table 1, fig.1).
#The work has been sponsored by the RFBR (Grant N 99-05-64908).
76
Table 1. Experimental gold solubility data, T=300oC, P=300 atm, pH start=2.88.
N order |
N run |
load CH3CSNH2 mg |
m H2S |
lgmH2S |
mAu .104 |
lgmAu |
pHcal. |
|||||
1 |
Au-92 |
56.3 |
0.05 |
-1.30 |
0.2386 |
-4.62 |
5.45 |
|||||
2 |
Au-93 |
112.7 |
0.1 |
-1.0 |
0.1895 |
-4.72 |
5.48 |
|||||
3 |
Au-94 |
225.4 |
0.2 |
-0.699 |
1.118 |
-3.95 |
5.49 |
|||||
4 |
Au-95 |
338.1 |
0.3 |
-0.523 |
8.46 |
-3.07 |
5.50 |
|||||
5 |
Au-96 |
450.8 |
0.4 |
-0.398 |
22.67 |
-2.64 |
5.50 |
|||||
6 |
Au-97 |
563.5 |
0.5 |
-0.30 |
29.4 |
-2.53 |
5.51 |
|||||
(4.69.10-2 mole/l Na2SO4) . 102 |
||||||||||||
7 |
Au-98 |
56.3 |
0.05 |
-1.30 |
0.03418 |
-3.48 |
7.5 |
|||||
8 |
Au-99 |
112.7 |
0.1 |
-1.0 |
0.1394 |
-2.855 |
7.4 |
|||||
9 |
Au-100 |
225.4 |
0.2 |
-0.699 |
0.4566 |
-2.34 |
6.9 |
|||||
10 |
Au-101 |
338.1 |
0.3 |
-0.523 |
1.239 |
-1.907 |
6.7 |
|||||
11 |
Au-102 |
450.8 |
0.4 |
-0.398 |
2.42 |
-1.616 |
6.56 |
|||||
12 |
Au-103 |
563.5 |
0.5 |
-0.30 |
2.61 |
-1.58 |
6.45 |
|||||
Fig.1. Partitioning of gold hydrosulphide complexes as a function of pH.
Fig.2. Gold solubility as a function of H2S concentration in the solution
References:
Dadze T.P., Orlov R.Yu. Thiocomplexes of antimony in a sulfide alkali solution from the Raman spectroscopy data.
Raman spectroscopy method has been used to study solutions of antimonite Sb2S3 in water Na2S solutions. Thiocomplexes, along with other sulfide sulfur complexes, are considered by many authors as important complexing agents participating in mobilization and transfer of gold in natural hydrothermal processes [1,2]. We determined antimony particles formed upon Sb2S3 dissolution in a water Na2S solution at temperatures 25-130oC by means of Raman spectroscopy. The concentration of antimony varied from 0.005 to 0.1 mole/l that of Na2S from 0.1 to 0.8 mole/l, the starting pH values ranged from 12.6 to 14. For all antimony concentrations the observable spectrum contained at least two RS lines: intensive well polarized line at 367 cm-1 and depolarized one, at 378 cm-1 frequency. At Na2S concentration in excess of 0.5 mole/l the third 350 cm-1 line was observed in the run whereas pure Na2S solution is characterized by a weak RS line with a frequency of about 314 cm-1. The found frequencies are practically coincident with the high-frequency RS spectral region of Na3SbS4 salt and its water solution in the work [3] where the polarized 367 cm-1 line and depolarized 380 cm-1 line were also observed. These lines correspond to totally symmetric and three-fold degenerated vibrations of the tetrahedral SbS43- group. The protonated antimony complexes were also calculated. The best fit of the calculated and observable frequencies was obtained for protonated HSbS4- and H2SbS4 complexes.
An experimental indication of the probability of a low-frequency wing (2574-2500 cm-1) with the 2574 cm-1 line related to SH- ion vibrations in the solvent.
References:
77