The basis for the fabrication of two-pyroxene geothermometer was provided by original experiments in the system CaO-MgO-FeO-SiO2 . It was assumed there that the presence of admixtures of other components (more than 5 wt%) can noticeably spoil its readings as, also, the use in the region of Mg-rich compositional (with 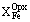 less than 0.1). The testing (table 1, Fig.1) has shown that a two-pyroxene geothermometer recommended in the program TPF can, without any significant errors, be employed also for minerals of high Mg-number and more complex composition. It has been found that deviations of the calculated values from experimental temperatures do not correlate with the mineral composition even if the calculation error grows as the mineral Mg-number increases.
less than 0.1). The testing (table 1, Fig.1) has shown that a two-pyroxene geothermometer recommended in the program TPF can, without any significant errors, be employed also for minerals of high Mg-number and more complex composition. It has been found that deviations of the calculated values from experimental temperatures do not correlate with the mineral composition even if the calculation error grows as the mineral Mg-number increases.
Fig.1. Testing of a two-pyroxene geothermometer [3] from the system of consistent geothemometers.
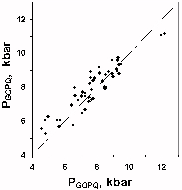
Fig.2. Comparison of Grt-Opx-Pl-Qtz (GOPQ) and Cpx-Pl-Qtz (GCPQ) geobarometers reading for natural garnet-two-pyroxene associations.
The statistical analysis (table 1) suggests that the known versions of two-pyroxene [2] and garnet-clinopyroxene [1] geothermometers are not advantageous, even in the region of rather high temperatures and pressures, against the versions recommended in the program TPF [3,8,4]. Moreover, the former geothermometer is ineffective in many cases, since it necessitates the solution of a system of non-linear equations, and the latter one has somewhat poorer characteristics at testing (Table 1).
The data on consistence of Opx-Cpx, Grt-Opx and Grt-Cpx geothermometers and the testing results for Grt-Opx-Pl-Qtz and Grt-Cpx-Pl-Qtz geobarometers were reported earlier [6,5,7]. P-T estimations were performed for natural complexes from Nilgiri (India) and Highland complex (Sri Lanka) using all these sensors and published analytical data (Fig.2 gives the composition of geobarometers readings). The testing confirmed high consistence of readings of both garnet-pyroxene geothermometers (N=43,  Tav.=24°C,
Tav.=24°C,  =41, R=0.889) and geobarometers (
=41, R=0.889) and geobarometers ( Pav.=-0.21 kbar,
Pav.=-0.21 kbar,  =0.62, R=0.926).
=0.62, R=0.926).
Gerya T.V. and Perchuk L.L. Hydrodynamic modeling of P-T paths for granulite facies terrenes.
Pressure-temperature (P-T) paths reflect movement and thermal regime in metamorphic evolution of a crystalline rock. They can be deduced from detail study of mineral equilibria in the rock and their evolution caused by change in P and T. In some rocks this evolution is resulted in the form of reaction textures in which reacting minerals show change in their compositions with T and P systematically. This systematic record of P-T parameters allows numerical modeling of the paths via monitoring of any 'sample' in the gravitational field. This modeling has been done in terms of hydrodynamic laws for metapelites collected from the Limpopo granulite belt situated among the Kaapvaal and Zimbabwe greenstone belts of southern Africa. The reaction textures in the rocks preserved both the isobaric cooling and the decompression-cooling regimes of exhumation. The calculations were done using the following data: densities (g/cm3) for sediments = 2.7, for metapelites = 2.8; for metabasites = 3.0, for komatiites = 3.3; viscosity contrast - 102; isobaric cooling limits are:  T=1000; boundary conditions: at top are Ttop = 300, K and dVõ=dVy=0, at bottom T = 1200, K, and Võ=Vy=0, at walls dT/dx= 0, dT/dy = const., and Võ=Vy=0, initial and final P-T parameters for the paths were used as the first approximation. Effective viscosity, 1019.5 poise, was computed in the course of numerical modeling. This value lies within limits, 1018 -1020 poises, used for the first modeling of gravitational redistribution of material in the Precambrian Earth's crust. As a result both types of P-T paths were reproduced for metapelites from the Limpopo high-grade terrain.
T=1000; boundary conditions: at top are Ttop = 300, K and dVõ=dVy=0, at bottom T = 1200, K, and Võ=Vy=0, at walls dT/dx= 0, dT/dy = const., and Võ=Vy=0, initial and final P-T parameters for the paths were used as the first approximation. Effective viscosity, 1019.5 poise, was computed in the course of numerical modeling. This value lies within limits, 1018 -1020 poises, used for the first modeling of gravitational redistribution of material in the Precambrian Earth's crust. As a result both types of P-T paths were reproduced for metapelites from the Limpopo high-grade terrain.
#Perchuk L.L., Yapaskurt V.O., Safonov O.G. Systematic chemical zoning of potassium-bearing pyroxenes as record of ultra-high potassium liquids in the earth 's mantle
The potassium-bearing very coarse-grained (megacrystal) diamond-free garnet-pyroxene rock occurs in the Kumdy-Kol microdiamond deposit that is situated in the Kokchetav massif, northern Kazakhstan. The deposit is composed of a variety of metasedimentary and magmatic rocks metamorphosed under amphibolite facies conditions at ~530 Ma. The quartz free rock studied forms interbeds and lenses in biotite-garnet gneisses near their contacts with calc-silicate rocks. Garnet of this rock contains micro-inclusions (from 50 to 185  m in size) of relict clinopyroxenes (Cpx1) with a high potassium content. No potassium-bearing minerals occur around Cpx1 inclusions. Analytical data suggest that potassium enters clinopyroxene as K jadeite (KAlSi2O6) resulting from the ultra-high-pressure isomorphic substitution KAlSi2O6 -Ca(Mg,Fe)Si2O6. A major constituent of the rock is potassium-free clinopyroxene (Cpx2). Cores and central portions of large (up to 90 mm in size) idiomorphic crystals of Cpx2 contain microcrystals of Kfs, while the Cpx2 rims are free of such inclusions. The Kfs microcrystals in Cpx2 form a lamellae-like texture that allows using the defocused beam for measuring of bulk composition of homogeneous clinopyroxene. The K2O content of both clinopyroxenes varies systematically from cores to rims (Perchuk et al., 1996; Perchuk & Yapaskurt, 1998).
m in size) of relict clinopyroxenes (Cpx1) with a high potassium content. No potassium-bearing minerals occur around Cpx1 inclusions. Analytical data suggest that potassium enters clinopyroxene as K jadeite (KAlSi2O6) resulting from the ultra-high-pressure isomorphic substitution KAlSi2O6 -Ca(Mg,Fe)Si2O6. A major constituent of the rock is potassium-free clinopyroxene (Cpx2). Cores and central portions of large (up to 90 mm in size) idiomorphic crystals of Cpx2 contain microcrystals of Kfs, while the Cpx2 rims are free of such inclusions. The Kfs microcrystals in Cpx2 form a lamellae-like texture that allows using the defocused beam for measuring of bulk composition of homogeneous clinopyroxene. The K2O content of both clinopyroxenes varies systematically from cores to rims (Perchuk et al., 1996; Perchuk & Yapaskurt, 1998).
Microprobe profiles across both clinopyroxenes show complex, but similar changes in potassium Mg/Fe ratio. K2O of Cpx1 decreases toward a rim grain from 1.05 to 0.47 wt. % each (Fig.1a), while that of Cpx2 is about 0.45 in core and centre of a megacryst reaching zero in the rim of the grain (Fig.1b). The chemical zoning of Cpx1 provides evidence for its crystallization before garnet under very deep mantle conditions from a liquid (l) that was very rich in potassium (Shimizu, 1974; Harlow, 1997). Since decrease in T and P, Cpx1 sharply loosed potassium till 0.45-0.47 wt. % at the moment of crystallization of garnet from a liquid. Cpx2 has been reacting with liquid that was oversaturated with potassium after the peritectic reaction KAlSi2O6 + [SiO2]m/fl = KAlSi3O8, i.e. Kjd + (silica from liquid) = San (lamellae in Cpx2). The Cpx1 micro-inclusions were isolated from the ascending liquid by garnet crystals being unable to react with the liquid anymore. The Kfs+Grt+Cpx intergrowth around garnet presumably crystallized at the freezing point. At the final stage of evolution, the rock experienced regional eclogite and then amphibolite facies metamorphism recorded in the (i) formation of the Ep-Kfs pseudomorphose after Cpx1, and (ii) Possible Ca Mg substitution in garnet: both resulted from the fluid-rock interaction. Ultrahigh-potassium liquids were found in diamonds from kimberlites (Table 1).
Mg substitution in garnet: both resulted from the fluid-rock interaction. Ultrahigh-potassium liquids were found in diamonds from kimberlites (Table 1).
Profiling across Cpx2+Kfs has been carried out with defocused beam in the CamScan and Camebax microbes. It is clearly seen that minimum of K in Cpx2 corresponds to maximum of potassium in Cpx2. Points P1 in both diagrams indicate beginning of decrease in pressure and temperature, and appearance of the first grains of Cpx2, while points P2 in the diagrams reflect peritectic reaction KAlSi2O6 + [SiO2]m/fl = KAlSi3O8, i.e. Kjd + (silica from liquid) = San (lamellae in Cpx2).
#Funding was provided by the RFBR grants 96-05-98470 to LLP.
62
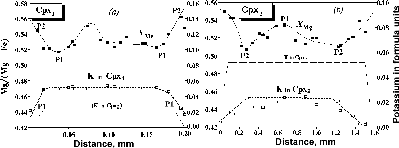
Fig.1. Compositional profiles across Cpx1 (a) from inclusion in garnet and Cpx2 (b) from the matrix clinopyroxene containing Kfs lamella from studied Cpx-Grt rocks.
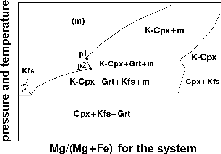
Fig.2. Schematic non-isobaric T-X melting diagram for the pseudobinary system garnet-potassium-bearing Cpx. The diagram was deduced from the microprobe profiling data of Fig..
Table 1. Metal oxides, water and caronate in microinclusion-bearing diamonds (Navon et al., 1988)
|
CTP |
CTP |
CTP |
CTP |
CTP |
CTP |
GRR |
GRR |
GRR |
GRR |
GRR |
GRR |
GRR |
GRR |
GRR |
Sample |
6268 |
L0 |
L6 |
LB |
Z4 |
MM1 |
1503 |
1504 |
1508 |
861.2 |
1155 |
1515 |
1517 |
1518 |
1519 |
Morphology |
Octahedrons |
Cubes |
Province |
Bots- wana |
Zair |
Uncertain source (probably Zair) |
Points |
4 |
5 |
2 |
2 |
3 |
2 |
7 |
8 |
10 |
5 |
10 |
7 |
3 |
2 |
2 |
Si02 |
31.9 |
41.2 |
43.3 |
34.6 |
67.7 |
40.4 |
35.6 |
42.3 |
42.4 |
51.1 |
53.6 |
45.1 |
30.3 |
42.4 |
45.9 |
TiO2 |
4.2 |
2.4 |
2.5 |
2.1 |
2 |
2.9 |
2.8 |
2.6 |
2.7 |
2.4 |
4 |
2.3 |
3.4 |
2.9 |
2.6 |
Al2O3 |
2.9 |
6.1 |
5.4 |
5.6 |
5.9 |
4.5 |
3.3 |
4.9 |
4.9 |
5.4 |
4.3 |
4.6 |
5.3 |
4.4 |
4.8 |
FeO |
15.7 |
5 |
5.6 |
4.9 |
3.3 |
7.2 |
8.3 |
11.1 |
6.1 |
6.8 |
6.6 |
10.1 |
5 |
8 |
8.8 |
MgO |
5.7 |
2.8 |
3.8 |
2.3 |
1.3 |
4.6 |
6.1 |
4.6 |
3.6 |
5.7 |
2.6 |
8 |
4.3 |
4.9 |
4.9 |
CaO |
10.5 |
10.7 |
10.6 |
12.3 |
1.6 |
13.9 |
16.8 |
7.8 |
11.9 |
8.6 |
7.2 |
7.6 |
18.7 |
9.8 |
12.4 |
Na2O |
2.6 |
3 |
2.9 |
3.5 |
1 |
3.8 |
2.9 |
2.3 |
2.4 |
2.1 |
1 |
4.8 |
2.7 |
3.4 |
3.4 |
K2O |
21.4 |
23.7 |
20.8 |
29.7 |
12.3 |
17.7 |
18.6 |
19.4 |
21.1 |
11.6 |
15.5 |
12.4 |
25.2 |
19.3 |
12.1 |
Oxides, ppm |
1195 |
433 |
211 |
247 |
1412 |
107 |
559 |
1207 |
508 |
80 |
551 |
628 |
22 |
118 |
99 |
H2O ,ppm |
407 |
191 |
118 |
140 |
294 |
165 |
282 |
619 |
269 |
98 |
168 |
241 |
|
|
148 |
CO2, ppm |
600 |
79 |
44 |
66 |
107 |
89 |
292 |
135 |
133 |
67 |
173 |
139 |
|
|
44 |
XflH2O* |
0.5 |
0.8 |
0.8 |
0.8 |
0.7 |
0.6 |
0.9 |
0.8 |
0.7 |
0.6 |
0.7 |
0.8 |
|
0.8 |
0.9 |
* XflH2O = H2O/ H2O + CO2, molar fraction.
63
References:
- Navon O., Hitcheon I.D., Rossman G.R., Wasserburg G.J. (1988) Mantle-derived fluids in diamond microinclusions. Nature. 325, 784-789.
- Perchuk L.L., Yapaskurt V.O. (1998). Deep-seated ultra-high potassium liquids. Geology and Geophysics, No 6
- Perchuk L.L., Yapaskurt V.O., Okay A. (1995) Comparative petrology of diamond-bearing metamorphic complexes. Petrology, 3, 267-309.
#Fonarev V.I., Konilov A.N. P-T parameters of rock metamorphism in the Nuliyam and Kunnanpara (Kerala Khondalite belt, India)
Bt-Grt gneisses with evolving spots, sometimes veins, of incipient charnockites are exposed in the Nuliyam. Massive charnockites and calc-silicate rocks are also present. It has been found that Fe-Mg minerals of all rocks varieties, except calc-silicate ones, have an extremely high iron content (xFe=Fe/Fe+Mg): Grt - 0.9-0.97, Bt-0.45-0.73, Opx- about 0.8. The compositions of minerals from massive charnockites, incipient charnockite spots and charnockite-bearing Bt-Grt gneisses are, practically, alike which indicates the absence of noticeable changes in the bulk rock composition (with respect to the major components) during their charnockitization. Opx and Pl are, in all the cases, compositionally homogeneous, Grt is usually also homogeneous and zone-free, but its iron content increases appreciably at contacts with biotite. The matrix biotite is homogeneous, at contacts with garnet the iron-content of the mineral noticeably decreases. A paragenetic analysis of minerals using the data of their microprobe analyses and petrographic relations, suggests, that charnockites (both massive and incipient), their host gneisses, and calc-silicate rocks, contacting with them, were metamorphosed under similar P-T conditions.
In the Kunnanpara leucocratic garnet-biotite gneisses sometimes with large (up to 2 cm) porphyroblasts of garnet, are prevailing rocks. They contain small (from several centimeters up to 1 meter) frequently boudinated folded bands of charnockites and melanocratic two-pyroxene basic granulites occur. In gneisses and even in basic granulites of the Kunnanpara minerals are appreciably more Mg-rich than in Nuliyam, though the contents of iron in them is rather high as well.
Two stages of metamorphism with the following mineral associations are evident (fig.1).
I . Bt (matr.) + Grt (cors) + Pl + Kfs + Qtz (in gneisses)
Bt (matr.) + Opx + Pl + Fsp + Qtz and
Grt + Opx + Pl +Kfs + Qtz (in charnockites).
II. Bt (rims) + Grt (rims) + Fsp + Qtz (in gneisses and in charnockites).
Quantitative estimations of P-T parameters of metamorphism performed with the use of systems of consistent mineralogic thermometers and barometers (TPF program) confirm the conclusion about at least two metamorphic events in the region: 1. T=730±35°C, P=3.56 kbar (charnockite formation) and T=697±40°C, P=5.36 kbar (host rocks metamorphism); 2. T=565°C, P=0.9 kbar. However, higher-temperature metamorphic events are not excluded for the region. The charnockites, both incipient and massive were formed during the stage M1 at slightly higher temperatures than host rocks, and at appreciably lower pressure. Main factor of charnockitization was an increase of potassium activity and partial pressure of CO2 in zones of local tectonic fractures, arising at a subisothermal decompression of the crust.
The experimental results [4] and their processing for Ti-bearing biotites show that fluid consisted mainly of CO2 (xCO2 = 0.75). This conclusion is fully consistent with the data on the fluid inclusions in metamorphic rocks of the region.
The available data on the absolute age determination are indicative of preferentially Pan-African metamorphism (0.5-0.55 b.y.). But there are enough grounds to suppose that the events in the region are more ancient: 1.096 b.y. [2] and ~1.8 b.y. [1]. As a whole the metamorphism depth in the investigated regions of the Kerala khondalite belt corresponds to the depth of the Pan-African metamorphism in the Eastern Ghats mobile belt [3]. In the first region, however, metamorphism proceeded under more gradient conditions (at higher temperatures). Possibly, it reflected the conditions of rifting, earth's crust opening accompanied by growth of rocks permeability and, therefore, intensive transfer of heat and fluid fluxes from the depth.
References:
- Bartlett J.M., Dougherty-Page J.S., Harris N.B.W., Hawkesworth C.J., Santosh M. (1998) The application of single zircon evaporation and model Nd ages to the interpretation of polymetamorphic terrains: an example from the Proterozoic mobile belt of south India. // Contrib. Mineral. Petrol., V.131, pp.181-195.
- Bindu R.S., Yoshida M. (1997) Dating of zoned monazite grains from south Indian granulites. // International Symposium "Origin and Evolution of Continents" in Tokyo, Japan, October 13-14, 1997. Program and Abstract, National Institute of Polar Research, p.10.
- Fonarev V.I., Konilov A.N., Rao A.T (1998) P-T conditions of polymetamorphism in the central part of the Eastern Ghats mobile belt, India. // Petrology, V.6, N.1, pp.70-85.
- Konilov A.N., Fonarev V.I. (1993) An experimental study of the charnockite assemblage biotite+orthopyroxene+K-Feldspar+quartz. // Petrology, V.1, N.3, pp.224-240
#The work has been sponsored by the INTAS (Grant 94-2466) and RFBR (Grants N 98-05-64563 and 98-05-64564).
64
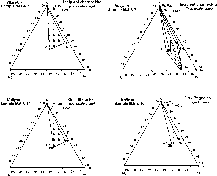
Fig.1. Mineral associations and compositions of co-existing minerals and hosting them Grt-Bt gneisses in the Nuliyam region (South India)
#Fed'kin V.V. Mineralogical geothermobarometry: scope for geothermal interpretation.
Methods of mineralogical geothermobarometry are widely employed in studies of metamorphic formations, providing the means of determination of evolution of physicochemical conditions of metamorphism in time and space. The T-P-trends of this process reflect to a certain extent, the geothermal gradient line position of the region under study in the course of the formation development. That, when different-age objects are studied, makes it possible to follow their displacement in the geologic history.
From this standpoint, using methods of physicochemical analysis of mineral paragenesis and mineralogic geothermobarometry, we have studied two Earth's crust fragments, different in age and structure-geological positions, from the Balkan Peninsula: the outcrop of the most ancient Palaeozoic crystalline base of the Serbo-Macedoninan Composite Terrane in the Batocina block, representing an ancient rather consolidate continental crust region, and the contact aureole around an oceanic crust fragment - the Bistrica block of ultramafite rocks in the Dinaridic Ophiolite Belt Terrane. These formations, not related in time and space, had a different history of geologic evolution, but the end stage of their metamorphic evolution proceeded under identical physicochemical conditions of the single earth crust fragment.
Mineral associations of amphibolites from the Batocina formation retain traces of progressive metamorphism of different depth level from t=430-530oC and P=5-8.2 kbar to t=620-680oC and P=9-10 kbar. Later retrograde changes in the compositions of co-existing minerals of staurolite schists of the same formation fix the parameters from the conditions of epidote-amphibolite (t=540-580oC and P=8-9 kbar) to greenschist (t=340-420oC and P=0.4-1.5 kbar) facies. The opposite trend of the metamorphic processes revealed in rocks of the same formation is associated with different ability of rocks-forming minerals to keep their composition and to fix the mineral formation conditions at different metamorphic stages.
Nevertheless, the both physicochemical conditions evolution trends of metamorphism of the formation lead, finally, to a single trend of change of the P-T mineral-formation parameters at its final (post-Mesozoic) stage which reflects, in our opinion, the geothermal gradient line position at that time.
The prograde metamorphism in contact aureole of ultramafitic bodies in the Dinaridic Ophiolite Belt Terrane gave birth to Grt-Cpx-Hb-Pl crystalline schists and garnet amphibolites. Prograde zoning, which reflects the initial strongest stage of metamorphism is preserved in central parts of rock-forming mineral grains. The edge zones of the coexisting phases (Grt, Cpx, Hb) fix in their composition the retrograde mineral formation stage with decreasing parameters from t=740-830oC and P=8-10 kbar to t=570-650oC and P=3.5-7.0 kbar. At the final stage the retrograde wave reaches the same parametric level (geothermal gradient lines) as the Batocina formation but in the opposite direction - from the side of higher T-P values.
#The work has been sponsored by the RFBR
(Grant N 99-05-64002).
65
So, the multistage evolution of metamorphism of two geologically different earth crust fragments - continental Batocina formation and oceanic Bistrica formations lead to the same physicochemical conditions of the final (post-Mesozoic) stage of their evolution.
#Fed'kin V.V., Kotova L.S., Kotov V.V. Allochemical transformations and conditions of formation of eclogite - glaucophane - schist rocks from the Atbashi complex, South Tien Shan.
The Atbashi metamorphic complex stands out in the Hercynian folded formation of the South Tien Shan as the Precambrian base, and is a part of the Kasan-Atbashi island arc embedded in the upper Ordovician-lower Silurian. The eclogite-bearing mass of metamorphic rocks - the Choloktor formation [3] - is located as a narrow 10-15 km long zone of a visible thickness 1.0-1.5 km with steeply sloping tectonic contacts with different - age complexes (from Proterozoic to upper Carboniferous) at the north-west of the Atbashi ridge. Its age is 320-360 my [2] although there are more ancient datings, i.e. 1100 and 550 my.
The Atbashi locates in the zone of joint of large structure-tectonic elements, namely, Middle and South Tien-Shan. It stands out among the host rocks in the level of metamorphism. Detailed petrographic studies of eclogite-glaucophane-schist and their satellite rocks in the formation have shown that their genesis is a result of combination of complicated metamorphic and metasomatic transformations of initial sediments under conditions of elevated pressure and moderate temperatures. The data on sequence of mineral transformations of high metamorphosed rocks of the complex (eclogite and eclogite-like garnet-clinopyroxene associations) to glaucophane schists and muscovite quartzite schists as well as petrochemical studies attest a gradual alteration of the rock chemical composition in the course of the formation evolution.
At the first allochemical transformation stage due to escape of bases (mainly Ca, to a smaller degree Fe, Mg, Na) pyroxene is replaced by glaucophane, zoisite or garnet (depending on the escaping components mobility ratio). The process is accompanied by intensive rock silicification and carbonation. The second stage is characterized by enhancement of silicification of glaucophane and zoisite rocks and the beginning of aluminum and, possibly, sodium escape. At the third stage at the background of quite mobile behaviour of silica there grows the activity of potassium which leads to rock muscovitization and abundant formation of quartzites and quartzite schists. At final stages, when a considerable amount of aluminium escaped, potassium feldspar forms instead of muscovite [3]/ Petrochemical studies of individual rock groups do not give an unambiguous answer of whether sodium plays as a dominant part in the formation of glaucophane associations.
Most likely, sodium as well as aluminum, at the background of intensive income of silicon, carbon dioxide, and potassium behaved more inert and only redistributed from one mineral phase to the other.
A microprobe study (more than 450 analyses) of the composition of the principal rock forming minerals, their zoning, composition of inclusions and direct contacts of co-existing phases has made it possible to reveal the specific features of evolution of metamorphism parameters in combination with allochemical rock transformation.
Both primary (garnet-clinopyroxene) and later (garnet-glaucophane) associations fixed in their composition their equilibrium growth temperature increase from grain center towards edge: from 500-550oC to 600-740oC and from 400-450oC to 515-600oC, respectively. "Prograde" zoning of garnet and the parameters of eclogite associations genesis reflect, possibly, the initial stage of tectonic evolution of the region under the conditions of rapid subduction and heating of the oceanic crust in the zone of its joint with continent. Retrograde mineral transformations of the formation rocks is accompanied by strong allochemical processes (glaucophane formation, muscovitization, carbonation, silicification, etc) are related to up-lift stage (island arcs or active continental margins) in the subduction zone. A close entangling of the processes of regressive stage of metamorphism and regional metasomatism of the acidic leaching facies determined the final appearance of the complex.
References:
- Bakirov A.B. (1984) Endogenic geologic formations of Kyrgyz. // In: Metamorphic formations, Frunze: Ilim, 216p.
- Dobretsov N.L. (1974) Glaucophane schist and eclogite - glaucophane - schist complexes in the USSR. // Proc., of the Institute of Geology and Geophysics, SB USSR Ac.Sci., Iss.57 Ed. V.S.Sobolev, SB Nauka Press, 430p.
- Kotova L.S. (1989) Petrochemical evolution of the Atbashi (South Tien Shan) eclogite - glaucophane - schist formation. // In: Geochemistry of magmatic and metamorphic formations of Tien Shan, Frunze: Ilim, pp.110-127.
Sultanov D.M., Maaskant P., Graphchikov A.A. TPF Sensor Manager Program.
The TPF program is used to calculate the mineral formation parameters (T,P, FO2) with mineralogic sensors (geothermometers, geobarometers, oxygen geobarometers, etc.). Due to simplicity and reliability, this program has become very popular with petrologists. Presently the program is complemented with new quite important features, namely, the possibility for a user to independently, add any sensors. These sensors can be used combined with the database containing more than 400 different versions of mineralogic thermometers, barometers, and their consistent system recommended by the authors [1,2,4,3].With this aim in view a new subprogram has been developed, i.e. TPF-SM (Sensor Manager) working in OS Windows 3.1. and higher. The system is simple and reliable which makes it possible to enlarge the scope of users of the TPF program. A convenient user interface of the TPF-SM enables every user to work with the program involving all the standard potentialities of editing (extracting, adding, altering, etc) for individual sensors, sensor groups, and componental molar fraction in minerals in accordance with a special list. The context-dependent "aid" structure eases the work with this program (figs. 1-3). Like in the largest number of the programs for OS Windows, a system of automated installation of the TPF program is realized in the TPF-SM. The TPF program is readily accessable in the Internet and can be found by address ftp: ftp.iem.ac.ru /pub/geology/tpf.zip
#The work has been sponsored by the RFBR
(Grant N 98-05-64002).
66
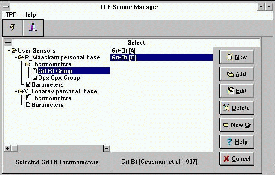
Fig.1. Examples of work with the TPF-SM. Creation of user (personal) sensor bases

Fig.2. Sensor editing. Sensor editor is performed as a blocknote. The page Minerals is used for additing, extracting, and edition the molar fractions of components in minerals from special list

Fig.3. The program has a context-dependent aid structure making it possible to call for aid at every program site.
67
References:
- Sultanov D.M., Maaskant P., Graphchikov A.A. (1998) Experiment in Geosciences , V.7,N.1.
- Fonarev V.I., Graphchikov A.A. In: (L.L. Perchuk, Ed.) (1991) Progress in metamorphic and magmatic petrology. A memorial volume in honor of D.S. Korzhinskiy. Cambridge University Press, pp.65-92.
- Fonarev V.I., Graphchikov A.A., Konilov A.N. (1991) Int. Geol. Review, V.33, pp.743-783.
- Fonarev V.I., Graphchikov A.A., Konilov A.N. (1994) Experimental studies of equilibria with minerals of variable composition and geologic thermobarometry. //Experimental Problems of Geology, Moscow: Nauka Press, pp.323-355. (in Russian)
- Graphchikov A.A and Fonarev V.I (1990) Garnet-orthopyroxene-plagioclase-quartz geobarometer (experimental calibration ). //Dokl. Akad. Nauk SSSR, V.312, N.5, pp.1215-1217 (in Russian).
#Dubinina E.O., Zharikova L.Yu. Model of the garnet zoning formation in the process of its recrystallization
At the present time the physicochemical conditions and durations of metamorphic processes are generally estimated by using the models suggesting the diffusion mechanism of zoning formation in garnet grains. The interpretation of these models is alleviated by the existence of quite a large number of experimental data on the diffusion coefficients of divalent cations in garnets. However, the proceeding of metamorphic reactions involving a change of both the mineral composition of the rock and the composition of co-existing minerals implies that recrystallization processes have to occur, also capable of leading to the formation of zoned garnet crystals. Therefore, experimental and theoretical searches for the criteria which could help to determine the zoning formation mechanism in natural garners is an actual problem for the study of dynamics and evolution of metamorphic processes. In terms of this problem, we carried out an experimental modelling of garnet zoning with the participation of divalent cations (Fe, Mg, and Mn) [1] and performed a preliminary analysis of the obtained data by means of theoretical modelling.
For conformity with the experimental conditions it was supposed, when creating the model, that:
- the system, is closed with respect to fluid;
- all the dissolved mass deposits in the form of the rim material in the course of recrtystallization, and
- the coefficients of divalent cations partitioning between the garnet and the fluid do not change throughout the process.
Description of the model. Designations:
Ñî(m) concentration of a component in the dissolving phase (or stoichiometric mixture) expressed as a fraction of the total mass of the dissolving materials;
Ñî(f) the same in the fluid before the onset of the dissolution;
m(f) fluid mass;
m mass of the redeposited solid phase in the form of rim;
Ñmean(m) mean concentration of a component in the rim;
Ñ(f) variable concentration of a component in the fluid with the growing rim;
k coefficient of an equilibrium partitioning of a component between the growing rim and the fluid;
Cp concentration of a component in the newly formed rim layer.
We assume that the rim growth on a garnet grain occurs at the expense of dissolution of some phase of the known composition involving a transfer of a cation of interest according to the scheme: dissolving phase  fluid
fluid 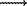 garnet. The total mass balance at every moment of such transfer process can be written as:
garnet. The total mass balance at every moment of such transfer process can be written as:
Ñî(m)m + Ñî(f)m(f) = Ññð(m)m + Ñ(f)m(f) (1)
We express Ñmean(m) as
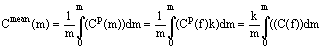 , (2)
, (2)
where 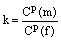 (3)
(3)
Substituting (2) into (1) and differentiating in m, we obtain
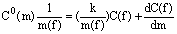 (4)
(4)
By solving eq (3) at the boundary condition C(f)=Co(f) with m=0, we obtain the dependence of the component concentration in the fluid on the mass of the newly formed rim.
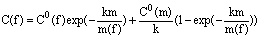 (5)
(5)
For the case of the rim growth on one crystal one can come from the variable m to the linear co-ordinate l which corresponds to the rim growth direction and is reckoned from the plane of the initial face in the normal to it direction towards the rim edge. Knowing the geometric parameters of garnet crystal we introduce the geometric factor g so that the equality m=lg be valid. In this case we can calculate a theoretical concentration profile of the component in the rim since with the partitioning coefficient being constant, it will correspond to the functional dependence (5) with account taken of (3). When a rim forms on large garnet crystals (when the thickness of the growing rim is small against the grain size), the geometric factor g can be equalized to the grain surface area S, taking into account the density of garnet, 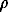 .
.
Allowing for the above and coming to the concentrations in garnet in terms of (3), we have
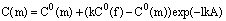 (6)
(6)
where A=(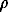 S)/m(f)
S)/m(f)
Taking the logarithm of (6), we obtain:
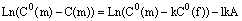 (7)
(7)
Analysis of the experimental data. The model calculation was tested on an experimental crystal of almandine with a Fe-Mg garnet rim, grown on it. As follows from eq(7) the natural logarithm, of difference of Fe or Mg concentrations in the charge and in the given rim point has to depend linearly on the distance l at which this point is away from the boundary of the unaltered garnet with the grown rim.
#The work has been sponsored by the RFBR
(Grant N 97-05-65383).
68
The partitioning of Fe and Mg in run (468) showed the correspondence with the theoretical model, and the linear dependence in accordance with eq (7) was found (fig.1).
In finding the parameters of linear regression of the logarithm of the difference in concentrations of the initial mixture and the garnet rim from the distance in the growth direction one can estimate the coefficient of the cation partitioning between the garnet and the fluid. Preliminary calculation from the results of concentration measurements along two profiles across the Fe-Mg zone yields the partitioning coefficients values KFe=1.6 and KMg=0.7. The coefficients of garnet-fluid partitioning were estimated in the same way for Fe and Mn when a Fe-Mn rim was growing (run 473). The values KFe=1.5 and KMg=1.7 were obtained for the two profiles.
These values show the degree of cation extraction from a solution at hydrothermal garnet growth. It is seen that in the presence of magnesium in the solution the degree of Fe extraction is higher than in the presence of manganese. Manganese extraction by the growing garnet is the strongest against other cations, magnesium is selected least effectively, its partitioning coefficient is less than 1. That is why magnesium is accumulated in the solution as the rim grows.
The obtained coefficients, as a whole, are positively correlated with the ion radius of divalent cations, and they can be arranged as a series KMg <KFe<KM n. It can be expected that for Ca the partitioning coefficient has to be still higher than for manganese since it has the largest ion radius in this series. Preliminary estimations show that the iron-magnesium garnet matrix catches a divalent ion of iron less effectively than the manganese one does. This fact is, however, to be refined.
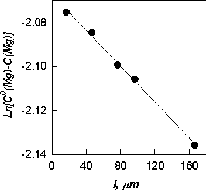
Fig. Natural lg of the difference between Mg concentration in the charge and that in the rim grown on almandine grain FeMg vs the distance from the measurement point relative to the Alm//Fe-Mg garnet boundary.
Reference:
- Zharikova L.Yu., Fonarev V.I. (1998) Zonal garnets: experimental and natural data. // Exp. In Geosci., V.7, N.1, pp.32-34.
69
Previous
Contents
Next
 ) of CO2 inclusions within a sample, grain and same fluid-inclusion trail is usually noticeable vary (in some cases from -31 to 8oC; -15.5 to 26oC, etc). These Th variations are likely related to the post trapping reequilibration of CO2 inclusions on retrograde PT-path. Most dense inclusions with Th=-35 and -23.6oC (
) of CO2 inclusions within a sample, grain and same fluid-inclusion trail is usually noticeable vary (in some cases from -31 to 8oC; -15.5 to 26oC, etc). These Th variations are likely related to the post trapping reequilibration of CO2 inclusions on retrograde PT-path. Most dense inclusions with Th=-35 and -23.6oC ( =1.097 and 1.049 g/cm3) were found in anorthosites; less dense (Th=18-25 oC and higher) homogenizing into the liquid phase are practically present in all the studied samples. Some enderbites contain secondary CO2 inclusions homogenizing into the gas phase (Th=27.5 oC and higher).
=1.097 and 1.049 g/cm3) were found in anorthosites; less dense (Th=18-25 oC and higher) homogenizing into the liquid phase are practically present in all the studied samples. Some enderbites contain secondary CO2 inclusions homogenizing into the gas phase (Th=27.5 oC and higher).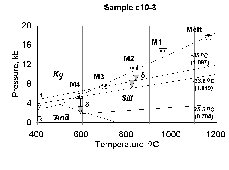
 -pressure differences between the stages M2, M4 and isochors 1 and 3, respectively.
-pressure differences between the stages M2, M4 and isochors 1 and 3, respectively.
 T);
T); 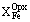 less than 0.1). The testing (table 1, Fig.1) has shown that a two-pyroxene geothermometer recommended in the program TPF can, without any significant errors, be employed also for minerals of high Mg-number and more complex composition. It has been found that deviations of the calculated values from experimental temperatures do not correlate with the mineral composition even if the calculation error grows as the mineral Mg-number increases.
less than 0.1). The testing (table 1, Fig.1) has shown that a two-pyroxene geothermometer recommended in the program TPF can, without any significant errors, be employed also for minerals of high Mg-number and more complex composition. It has been found that deviations of the calculated values from experimental temperatures do not correlate with the mineral composition even if the calculation error grows as the mineral Mg-number increases.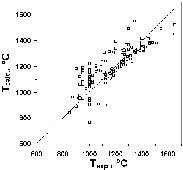
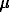 m in size) of relict clinopyroxenes (Cpx1) with a high potassium content. No potassium-bearing minerals occur around Cpx1 inclusions. Analytical data suggest that potassium enters clinopyroxene as K jadeite (KAlSi2O6) resulting from the ultra-high-pressure isomorphic substitution KAlSi2O6 -Ca(Mg,Fe)Si2O6. A major constituent of the rock is potassium-free clinopyroxene (Cpx2). Cores and central portions of large (up to 90 mm in size) idiomorphic crystals of Cpx2 contain microcrystals of Kfs, while the Cpx2 rims are free of such inclusions. The Kfs microcrystals in Cpx2 form a lamellae-like texture that allows using the defocused beam for measuring of bulk composition of homogeneous clinopyroxene. The K2O content of both clinopyroxenes varies systematically from cores to rims (Perchuk et al., 1996; Perchuk & Yapaskurt, 1998).
m in size) of relict clinopyroxenes (Cpx1) with a high potassium content. No potassium-bearing minerals occur around Cpx1 inclusions. Analytical data suggest that potassium enters clinopyroxene as K jadeite (KAlSi2O6) resulting from the ultra-high-pressure isomorphic substitution KAlSi2O6 -Ca(Mg,Fe)Si2O6. A major constituent of the rock is potassium-free clinopyroxene (Cpx2). Cores and central portions of large (up to 90 mm in size) idiomorphic crystals of Cpx2 contain microcrystals of Kfs, while the Cpx2 rims are free of such inclusions. The Kfs microcrystals in Cpx2 form a lamellae-like texture that allows using the defocused beam for measuring of bulk composition of homogeneous clinopyroxene. The K2O content of both clinopyroxenes varies systematically from cores to rims (Perchuk et al., 1996; Perchuk & Yapaskurt, 1998).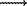 Mg substitution in garnet: both resulted from the fluid-rock interaction. Ultrahigh-potassium liquids were found in diamonds from kimberlites (Table 1).
Mg substitution in garnet: both resulted from the fluid-rock interaction. Ultrahigh-potassium liquids were found in diamonds from kimberlites (Table 1).

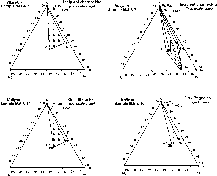



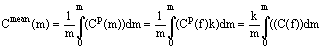 , (2)
, (2) 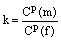 (3)
(3)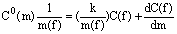 (4)
(4)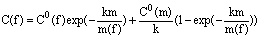 (5)
(5)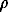 .
.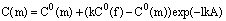 (6)
(6)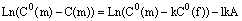 (7)
(7)