III. Metasomatism and modelling of the ore formation
(Leader Prof. G.P.Zaraisky)#Aksyuk A.M. Experimental study of melting conditions for albitites, potash feldspatites, granites and greisens composing the line formation in rare-metal granites in the Orlovka tantalum deposit.
The line formation incorporating quartz-albite, quartz-potash feldspar (amasonite) and quartz-mica ("greisen") layers is extensively grown in domes of Li-F granites stocks in the Orlovka tantalum deposit (East Transbaikalia) [1,2,3,4]. Whether the natures of these layers is magmatic or metasomatic is open to question. The melting experiments on rock samples from these layers were aimed at solving this problem. The onset of melting of Li-F granite (sample A-92), albitite (sample Ш-27/3), quartz-amasonite pegmatoid (sample З-19/2), and "greisen" (sample Ш-27/1) was experimentally studied on natural rock samples from the Orlovka deposit. Muscovite-albite topaz-bearing granite (sample A-92) contained (in vol%) quartz (25-30), muscovite (5-8), topaz (3-5). The composition of albitite ( sample Ш-27/) was: quartz (40-50), albite (20-25), muscovite (5-10), potash feldspar (1-5), beryl (5-8). The composition of pegmatoid (sample З-19/1) was: quartz (15-20), amasonite (40-50), albite (15-20), muscovite (5-7), topaz (1-3). Greisen was composed of quartz (40-50), mica (20-30), albite (20-30), potash feldspar (1-5), beryl (5-8).
The ground rock (30 mg) was loaded and sealed in a platinum capsule with added 0.1 m HF solution under preset experimental TP conditions so that the run conditions should correspond to water-saturated ones. The run duration was 2-8 days, depending on the experimental T-P conditions. The melting was studied in the range of 550-800oC and 0. 5-3 kbar. The solidus position was defined by the first advent of glass that cemented the initial powder. Inasmuch as the total quantity of fluid did not exceed 17%, and fluorine goes preferentially into the melt under the experimental conditions, the solubility of the solid charge in the fluid could not change noticeably the initial compositions of rocks in question. The additional amount of fluorine in the solution (0.66-1.02) wt % also did not affect significantly the additional solubility of water fluorine-bearing melts, the studied T-P conditions of solidus correspond to water-saturated ones. The obtained glass was colourless or greenish with inclusions of small "bubbles" formed upon quenching which is also indicative of the melt fluid-saturation under the run conditions.
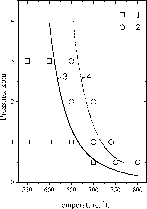
The obtained experimental data have shown that all the rocks from the line formation of the Orlovka granites, i.e. granites, albitite, potash feldspar, and "greisen" start melting under close T-P conditions (fig.1). The rocks solidi T-P parameters were: at 0.5 kbar 700-750oC, at 1 kbar 650-700oC, at 2 kbar 620-650oC, at 3 kbar 600-630oC. These solidi parameters were approx. 60oC lower than the solidus of haplogranite which is primarily related to enrichment in fluorine of the Orlovka rocks.
The close conditions of the melting onset for rocks from the line formation support our field observations concerning the magmatic nature of this complex. Apparently, the line rocks differentiated from Li-F granite melt before the crystallization of the basic part of the granitic stock and formed thin bodies in the crystallised upper "crust" of the Li-F granite stock dome or in the host schist strata.
Fig.1. Experimental data on the solidi T-P parameters for line rock formation (Li-F granites, albitites, pegmatoids, "greisens") in granites of the Orlovka tantalum deposit: 1-melting is absent; 2- melting is present, 3-solidus line of the Orlovka granites; 4- solidus line of haplogranite (Manning et al., 1984)
References:
#The work has been sponsored by the RFBR (grants 99-05-64109 and 98-05-64559).
41
Aksyuk A.M. Fedkin A.V., Seltmann R. Micas from granites of the Orlovka and Etyka tantalum deposits and estimations of HF concentrations in endogenic fluids.
Micas, due to versatility of their composition, cation and anion isomorphism reflecting both the medium and physicochemical conditions of their formation, are carriers of manifold though difficult to interpret genetic physicochemical information. A study of micas in the Orlovka and Etyka tantalum deposits, Eastern Transbaikalia, is important for understanding physicochemical conditions of rock formation in these deposits, including such specific ones as granitic line rocks and so-called ''apogranites".
Micas from the Orlovka and Etyka tantalum deposits are clearly divided compositionally into three groups of solid solutions: biotite, muscovite-Li-muscovite, zinnwaldite-cryophyllite-lepidolite series. They are characteristic, sometimes essential, constituent of not only amasonite granites but, also, of layered granitoid rocks developed in Orlovka and Etyka consisting of albitite, potash feldspatite and "greisen" layers. The compositions of micas from the Orlovka and Etyka rocks were studied by means of microprobe analysis. Over 1000 counts were performed on 30 samples of all most typical rocks, including multilayered granites.
Micas of parental granites. Subalkaline biotite leucogranites from the Khangilai and Oldandin plutons, taken as parental ones for the Orlovka and Etyka granites, respectively, contain about 5 vol% biotite and 3 vol% muscovite. Biotites, in the (Mg+Fe)/Al/Si ratio, are close to siderophyllite-eastonite series and are similar to biotite grown in contact hornstones. Interesting is that, when compared with biotites from the classic greisen Akchataus W-Mo deposit, they appear close, in this ratio, to biotites of II and III phase of the Akchataus granites which, additionally, indicates that the formation of Ta-Nb deposits necessitates much dipper differentiation of a granitic melt in the parental deep chamber than is typical of greisen deposits. Muscovites (Li-muscovites) in the Khangilai and Oldandin granites can contain up to 5-6% of iron, as recalculated to FeO, 0.5-1% TiO2, and 1-2% Fe.
Micas of muscovite granites. Coarse-grained porphyry-like muscovite granites from the Orlovka deposit, opened deep by boreholes, are analogous to granites from the Spokoiny W deposit located several kilometres away from Orlovka and have no analogs in Etyka. Muscovites (Li-muscovites) of these granites contain 2.5-5% FeO, traces of TiO2 (0.5%), and 0.5-1.5% F. Micas of the Spokoiny granites are compositionally close to pure muscovites.
Micas of ore-bearing granites. Ore-bearing topaz-containing amazonite, amazonite-albite granites from the Orlovka and Etyka most often contain two micas: (1) muscovite-Li-muscovite and (2) zinnwaldite-cryophyllite-lepidolite series. The former are dominant in the Orlovka granites, the latter more often cryophyllite, in the Etyka granites.
Micas of the line rocks. In layered granitoid rocks of Orlovka Li-muscovite-zinnwaldite micas dominate. In Etyka, where analogous rocks are developed without "greisen" layers dominate zinnwaldite - cryophyllite-lepidolite micas.
Estimation of fluorine concentrations in natural fluids. Inasmuch as thermodynamic properties and the affinity to fluorine of lithium-bearing micas are studied insufficiently, we have attempted to estimate them in the first approximation via the effect of Li on partitioning of fluorine between the mica and the fluid by modifying a biotite geofluorimeter [1,2] and estimating HF concentrations in the fluid related to the Orlovka and Etyka granites. Rough estimations of formation temperatures of micas in these granites yield somewhat different values for different granites in the range 750-620oC.Under the assumption that the rocks formed at pressures close to 1 kbar, according to the modified mica geofluorimeter, HF concentration in the magmatic fluid of even Khangilai biotite granites-parential granites for the Li-F Orlovka granites - was high and could amount to 1.5-2 m. Possibly, these are overestimates because of a too rough account of the effect of Li in the mica compositions but, apparently, high concentrations of HF in the fluid, mineralization and formation of line rocks in apical parts of the Orlovka and Etyka granitic domes are closely related.
References:
#Zaraisky G.P, Stoyanovskaya F.M., Tikhomirova V.I. Investigation of albite solubility in HF solutions at T=500oC and P=1 kbar.
In order to quantitatively estimate physicochemical conditions of albitization of lithium-fluorine granites, which is so typical of the Orlovka and Etyka tantalum deposits, we have continued albite solubility studies at T=500oC and P=1 kbar as a function of a change in the HF concentration in a solution ranging form 10-5 to 10-1 m. The volume of HF solution in a platinum autoclave insert was 10.75 cm3 (under normal conditions),
#The work has been sponsored by the RFBR (project N 99-05-64106) and INTAS Ref. No. 97-0721.
42
the run duration was 330-400 h. The total albite solubility was determined by weighing the single crystal before and after the run in grams with the accuracy to the 5-th symbol after the point. After the runs the solutions were analysed for SiO2 using a method of photometric determination of silico-molebdenum acid by the yellow complex, for Na-ion a chemico-analytic method using criochromcyanin-R. The values of total albite solubility, calculated from the chemical analysis data appear, on an average, 1.3 times lower than those determined from the weight loss which can be attributed to precipitation of some part of substance from the solution in the process of quenching. The obtained results are given in the table and the figure.
HF, concentr., mole/kg H2O |
Weight loss of Ab, g |
Total solubility of Ab, g/litre |
Concentration in the solution, mole/kg H2O |
Solubility of Ab from chem. analysis (cal.), g/litre |
||||
SiO2 |
Al3+ |
Na+ |
||||||
10-1 |
0.0546 |
5.079 |
0.0520 |
0.0096 |
0.0170 |
4.137 |
||
10-2 |
0.0150 |
1.395 |
0.0093 |
0.0021 |
0.0041 |
0.792 |
||
10-3 |
0.0093 |
0.865 |
0.0088 |
0.0020 |
0.0022 |
0.698 |
||
10-4 |
0.0088 |
0.818 |
0.0082 |
0.0014 |
0.0017 |
0.661 |
||
10-5 |
0.0078 |
0.726 |
0.0067 |
0.0009 |
0.0039 |
0.569 |
||
Fig. Solubility of albite vs the HF concentration at T=500oC and P=1 kbar.. a - total solubility in g/l determined from crystal weight loss and calculated from the chemical analysis data. b - concentration of SiO2, Al3+, and Na+ in the solution, mole/kg from the chemical analysis data.
The data given show that albite dissolves in HF with a preferential transition of silicon into the solution, with respect to its stoichiometric content in the mineral. This deviation is, however, small, rather consistent throughout the investigated range of HF concentration (from 10-5 to 10-1 mole/kg H2O), and is, on an average, about 30%. Note, that in the run with 0.1 m HF the concentration of SiO2 is higher than the saturation in quartz under the same conditions which may be associated with the formation of amorphous silica at acidic decomposition of albite. At the same time at lower HF concentrations the solution is SiO2-undersaturated with respect to quartz. Interestingly, that the concentration of Al3+ in 0.1 m HF solution corresponds to saturation in corundum, and in 10-5-10-2 m HF it appears even higher than an equilibrium with corundum under analogous condition [3]. The total solubility of albite measured from the weight loss increases monotonously from 0.726 to 1.395 g/l as the concentration of HF grows in the range from 10-5 to 10-2 m, and then drastically increases to 5.07 g/l in 0.1 m HF solution. The increase in the albite solubility occurs herewith not only due to a growth of silica concentration whose contribution to the total solubility of albite is maximal but it also found for Al and Na.
The obtained data suggest that in the range of HF concentrations 10-2-10-1 mole/kg H2O the total solubility of albite is quite comparable with the solubility of quartz in water, being 2.4 g/l at T=500oC and P=1 kbar [2]. In the 10-2 m HF it is somewhat lower than the solubility of quartz (1.395 g/l) but more than twice as much than the latter at an increase of the HF concentration to 10-1 m (5.079 g/l). So, one can conclude that under the conditions of moderate fluorinity quite real for postmagmatic solutions generated by lithium-fluorine granites [1], albite can dissolve and redeposit hydrothermally in amounts not smaller than quartz.
References:
43
#Ivanov I.P., Karadzhanov M., and Kanazirsky M. Quantitative analysis of the ore mineral parageneses in the system Fe-Cu-S-H2O-O2, open to sulfur and oxygen at T=300, 400oC and P=1 kbar
The quantitative analysis of ore mineral parageneses in the system Fe-Cu-S-H2O-O2 is performed. The diagrams of lg mStotal versus lg fO2 at T=300, 400oC and P=1 kbar are plotted in Fig.1 (mStotal the total sulfur concentration in solution (mole/kg H2O) and fO2 the fugacity of oxygen (bars)). These diagrams include: pyrite (Py), pyrrhotite (Po), magnetite (Mag), hematite (Hem), covellite (Cv), chalcocite (Cc), natural copper (Cop), cuprite (Cup), chalcopyrite (Ccp), and bornite (Bn). The calculations are performed with the application of Yu.V. Shvarov's software and database 'UNITHERM' [Shvarov, 1992].
The stability fields of double parageneses of the ore minerals are shown on the diagrams:
(1) Py + Cc; (1') Py + Bn, Bn + Cc; (2) Py + Ccp, Ccp + Bn, Bn + Cc; (3) Po + Ccp, Ccp + Bn, Bn + Cc; (4) Mag + Ccp, Ccp + Bn, Bn + Cc; (4') Hem + Ccp, Ccp + Bn, Bn + Cc; (5) Mag + Bn, Bn + Cc; (5') Hem + Bn, Bn + Cc; (6) Mag + Cc; (7) Hem + Cc; (8) Mag + Cop; (9) Hem + Cop; (10) Hem + Cup. The sulfur concentration was controlled by fugacity fS2, gas.
The compositions of solutions in equilibrium with mineral parageneses are calculated and extremum of S, Fe, and Cu concentrations in solution is determined. The steep inflection of solution pH values is observed. The role of temperature is demonstrated on the diagrams. The extremum is associated with the transition of predominant H2S(aq) particle to HSO-4 and SO2 particles (oxidation).
Fig.1. Diagrams of the Fe-Cu-S-H2O-O2 system at P=1 kbar. 1-10 indicate the stability fields of double parageneses. I-VII are the nonvariant points. A, B, and C represent the reference points. Dashed lines stand for the boundaries of fields in the end systems.
Korzhinskaya V.S. Experimental study of baddeleyite (ZrO2) solubility in the carbonate solutions at T = 200-400oC and P = 1 kbar
The new type of fine-dispersed tungsten-zirconium ores represented by baddeleyite and helzircon and deposited in the Vend dolomite marbles in the north of Aldan shield has been thoroughly investigated. It suggests determination of not only stability of minerals (zircon, quartz, and baddeleyite) under physicochemical conditions of their formation but also the forms of zirconium transport in the fluid.
The important role in zirconium migration is attributed to carbonate complexes. At present, however, the data on quantitative characteristics of equilibrium complex formation of zirconium with carbonate and bicarbonate ions are scanty.
1. A study of zirconium complex formation with carbonate and bicarbonate ions and baddeleyite solubility in Na2CO3 and NaHCO3 solutions in the concentration range 0.01 to 2 mole/kg H2O at T = 200, 300, and 400oC and P = 1 kbar is initiated. It is derived, that baddeleyite solubility in NaHCO3 is higher than in Na2CO3 by 0.5 - 1.0 logarithmic units (for instance, for Bd at T = 300oC, lgmZraq = -3.15 in 0.1m NaHCO3 and lgmZraq = -4.01 in 0.1m Na2CO3). The maximal baddeleyite solubility is observed at 0.1m concentration of NaHCO3. The compositions of complexes, formed in solution, were identified by IR-spectrometry.
2. A set of experiments in HCl solutions with NaCl additions was performed at T=500oC and P=1 kbar to investigate an influence of Cl-ion on the baddeleyite solubility in a chloride fluid. The NaCl concentration was used as 0.1, 0.5, 1, and 2 mole/kg H2O. Experiments have shown that NaCl addition up to 0.5m decreases the baddeleyite solubility and for 0.01 and 0.1m HCl concentrations it is coincident with solubility of ZrO2 in pure water (lgmZraq = -5.08) (see Fig.1). The solubility sharply decreases with increasing in NaCl concentration (up to 2m). This decrease may be explained by NaCl hydrolysis, which is the cause of changes in mHClovalue, whereas in the concentrated HCl solutions (1m) the hydrolysis of NaCl slightly affects the mHClo changes. Hence it is established that additional salt loading of NaCl slightly influences the equilibrium zirconium concentration in solution in the chloride fluid.
3. The free Gibbs energies of Zr hydroxicloride complex formation are calculated from our experimental data on baddeleyite and zircon solubility in the chloride fluid at T=400-600oC and P=1 kbar and HCl concentration range from 0.01 to 4 mole/kg H2O (see Table).
4. On the basis of our experimental data on concentrations of Si in equilibrium with baddeleyite-zircon association (Bd + H4SiO4o = Zrc + 2H2O) (1) and Zr in equilibrium with zircon-quartz association (Zrc + H2O +HClo = Zr(OH)3Clo + Q) (2) at mHCl = 1 mole/kg H2O the free Gibbs energy of zircon (ZrSiO4) is evaluated from the two independent equilibria (1) and (2) for T=400-600oC and P=1 kbar. The values of ZrSiO4 free Gibbs energy are presented in Table.
5. A computer calculation of fluid composition satisfying experimental conditions for sheelite-hubnerite equilibrium in the chloride solution at T=300-500oC is performed under GIBBS algorithm (software of Yu.V. Shvarov). The hubnerite (MnWO4) free Gibbs energy is calculated from three independent equilibria:
CaWO4 + Mn+2 = MnWO4 + Ca+2;
CaWO4 + MnCl+ = MnWO4 + CaCl+;
CaWO4 + MnCl2o = MnWO4 + CaCl2o .
#This work was supported by a grant N 99-05-64908 of the Russian Foundation for Basic Research.
44
lg m NaCl
Fig.1. Baddeleyite solubility in HCl+NaCl solutions at T=500oC and P=1 kbar.
Table.
Complex, mineral |
- |
|||
300oC |
400o C |
500oC |
600oC |
|
ZrSiO4 |
426.944 |
417.709 |
408.886 |
|
MnWO4 |
305.636 |
311.680 |
311.075 |
- |
Zr(OH)4o |
304.407 |
288.429 |
276.856 |
|
Zr(OH)3Clo |
283.560 |
271.431 |
259.492 |
|
Zr(OH)2Cl2o |
259.560 |
248.113 |
237.316 |
|
Zr(OH)Cl3o |
235.548 |
225.042 |
214.620 |
|
ZrCl4o |
211.291 |
201.618 |
191.605 |
|
References:
Redkin A.F. Solubility and fractionation study of some ore elements (W, Sn, U) in an H2O-NaCl fluid at 400oC and in the presence of an albite-andalusite-quartz buffer at 500oC.
The behaviour of W, Sn and U in the system WO3-SnO2-UO2-NaCl-H2O has been studied at 400oC, 200, 250 bars and a Fe2O3-Fe3O4 oxygen buffer. The initial WO3, SnO2 and UO2 are found not to suffer any noticeable changes. At T=400oC and P=200-250 bar tungsten redistributes preferentially to the L-phase, like a largest number of ore elements. The solubility of WO3 in the L-phase is 0.02+0.01 mole/kg H2O (at 500oC, 500 bar mLW=mVW= =0.07+0.03 mole/kg H2O).
SnO2 and UO2 have a lower solubility against that of W, and ore elements accumulate preferentially in the L-phase. At 400oC and P=250 bar mLSn and mLU are (2.0+0.8).10-4 and (1.4+0.5).10-5 mole/kg H2O, at P=200 bar, respectively, (2.3+0.2).10-4 and (2.4+0.2).10-5 mole/kg H2O. The partition coefficient of U, between the L and V-phases is 5<Kp=mLU/mVU<10. The solubility of SnO2 and UO2 is 1-2 orders of magnitude lower than at 500oC and P=400-500 bar where the acido-basic interaction of the components took place (the reaction WO3(S) + xNaCl + 0,5xH2O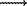 NaxWO3(S) + xHCl + 0,25xO2) and the solution itself was therefore more acid.
NaxWO3(S) + xHCl + 0,25xO2) and the solution itself was therefore more acid.
Preliminary experimental studies on solubility and fractionation of W, Sn, U and also, Fe and Si in a в NaCl-H2O-HCl fluid have been performed under the conditions where the solution acidity was specified by an Al-Si buffer, albite-andalusite-quartz one, and f(O2) HM (hematite-magnetite) and QFM (quartz-fayalite-magnetite) buffers at 500oC, 400, 500 and 1000 bar. It has been found that iron-bearing oxides readily dissolve in high-concentrated NaCl-HCl solutions. As magnetite dissolves, HCl is spent that causes the replacement of andalusite and quartz by albite so that m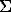 Na/ m
Na/ m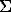 HCl=const. The concentration of Fe in the L-phase is approximately 0.1 mole/kg H2O. WO3 under these conditions is completely replaced by ferberite FeWO4. Due to high content of Fe in the solution the solubility of tungsten in the L-phase in the form of H2WO4 is considerably suppressed and makes up 3.10-5 mole/kg H2O. So, despite high concentrations of NaCl and relatively high acidity of the solution, the solubility of W from wolframite (ferberite) is suppressed by high solubility of Fe.
HCl=const. The concentration of Fe in the L-phase is approximately 0.1 mole/kg H2O. WO3 under these conditions is completely replaced by ferberite FeWO4. Due to high content of Fe in the solution the solubility of tungsten in the L-phase in the form of H2WO4 is considerably suppressed and makes up 3.10-5 mole/kg H2O. So, despite high concentrations of NaCl and relatively high acidity of the solution, the solubility of W from wolframite (ferberite) is suppressed by high solubility of Fe.
Solubility of cassiterite at the acidity dictated by the albite-andalusite-quartz buffer is lower than the sensitivity of the analytic determination technique. For UO2 a notable solubility is found only in the homogeneous NaCl-H2O-HCl region. The maximal solubility at 500oC and 1000 bar takes place in 40% NaCl and is 1.7.10-4 and 1.4.10-2 , respectively, for QEM and HM buffers.
45
#Chevychelov V.Yu., Zaraisky G.P. Experimental study of solubility of tantalite and limits of Ta incorporation into melts of rocks from the Orlovka tantalum deposit in Transbaikalia: effect of compositional alteration (Li-F granite, albitite, quartz-amazonite pegmatoid) at T=820oC, P=1 kbar.
Solubility of columbite-tantalite (one of the basic ore minerals) has been studied experimentally in melts of both individual layers of banded rocks and massive Li-F granites from the Orlovka tantalum deposit in Eastern Transbaikalia [1]. For the runs one most typical specimen was taken out of each of the four rock types: Li-F granites, albitite (albite-rich), amazonite (microcline) - rich, greisen-like layers with aplite or pegmatite texture [2.] The specimens were used to prepare Li- and F-rich homogeneous glasses. For that purpose the specimens were ground to powder , =0.6. wt % LiF powder and =5 % 0.2 m HF solution were then added and all was packed into Pt capsules. The melting runs were conducted in an internally heated gas pressure vessel at T=1050oC, P=1 kbar, and duration of about 1 day. The glass was then extracted, crushed and ground in a mortar. The glass powder was again packed into a Pt capsule after which the melting run was repeated under the same conditions for one more day. X-ray powder diffraction and microprobe analyses suggested that produced glasses were completely amorphous phases, quite homogeneous in the chemical composition. The glasses contained no less than 1 wt% F and about 2.5-4 wt% H2O.
Crystals of natural columbite-tantalite were used in solubility runs. A columbite crystal was located into glass powder and 0.2 m HF solution was added. The runs were conducted in a Tuttle bomb with water as pressure medium at T=820oC, P=1 kbar, the duration of the runs was 15-25 days.
Mean chemical compositions of the glasses after the solubility runs at =15 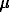 m from the boundary with the columbite-tantalite crystal are listed in the table (the microprobe analysis data).
m from the boundary with the columbite-tantalite crystal are listed in the table (the microprobe analysis data).
Li-F granitic glass (gr) |
Albite-rich glass (ab) |
Amazonite-rich glass (kf) |
Greisen-like glass (gs) |
|
SiO2 |
73.9 |
73.7 |
74.2 |
81.6 |
TiO2 |
0** |
0.1** |
0.1** |
0.1** |
Al2O3 |
16.0 |
17.3 |
15.4 |
12.6 |
FeO* |
0.3 |
0.4 |
0.1** |
0.2 |
CaO |
0** |
0.3 |
0.1 |
0.2 |
Na2O |
4.4 |
6.2 |
3.2 |
2.4 |
K2O |
4.4 |
1.3 |
6.2 |
2.2 |
Mn |
0.13 |
0.09 |
0.08 |
0.145 |
Ta |
0.18 |
0.125 |
0.14 |
0.10 |
Nb |
0.16 |
0.08 |
na |
na |
A |
1.30 |
1.34 |
1.23 |
1.76 |
*Total iron as FeO; ** less than 2 standard deviation;
standard deviation;
A - Molar. Al/(Na+K+2Ca).
Mean contents of tantalum in the melts were from 0.10 to 0.18, of Nb - 0.08-0.16, Mn - 0.08-0.15, and Fe - 0.1-0.3 wt%. The contents of the first two elements exceed significantly the starting ones (0.01-0.06 wt% Ta and 0.004 - 0.02 wt% Nb) but the contents of Mn and Fe did not practically change in the course of the runs. Fig.1 illustrates the contents of tantalum and manganese simultaneously measured in each point with 2 crystal-diffraction spectrometers of the microprobe. The maximum concentrations of tantalum were obtained in the granitic melt, the minimum in the greisen-like one, and in the albite- and amazonite-rich melts the tantalum concentrations had intermediate values. The direct proportionality was obtained for concentrations of tantalum and manganese for all the melts but for albite-rich one (from the data of 5-10 points of analysis for each melt composition). The diffusional distribution profile of tantalum was measured in a Li-F granitic melt for which the contents of tantalum were analysed in 15 points distant from the columbite crystal boundary at 10, 15, 20 etc to 400  m. The approximation of these analyses with the equation y=0.08+3.9/(x+27) yields =0.22 wt% Ta in glass at the boundary with columbite.
m. The approximation of these analyses with the equation y=0.08+3.9/(x+27) yields =0.22 wt% Ta in glass at the boundary with columbite.
Our solubility values for columbite-tantalite, (Mn, Fe)(Nb,Ta) 2O6 are quite comparable with the data of Linnen and Keppler (1997) [3] who studied the solubility of synthetic purely manganese compounds MnTa2O6 and MnNb2O6 in synthetic granitic melts of various composition (the molar Al/(Na+K) of the starting melt 0.64-1.22) at a pressure 2 kbar in the temperature range 800-1035oC. From the data of Linnen and Keppler, in figs. 2 and 3 are shown the effects of temperature and the molar Al/(Na+K+2Mn, Ca) on the solubility products (Ksp) of MnTa2O6 in granitic liquids, the solubility products (Ksp) of (Mn,Fe)(Nb,Ta)2O6 calculated from our data are represented as mean values for each of the three different rock types. The agreement of our data for Li-F granite (gr), albite-rich (ab) compositions with the data of Linnen and Keppler seems to be too good despite the pressures, compositions, etc differences in the experimental conditions.
So, it has been found that the limits of tantalum incorporation into Li-F granitic, albite-, and amazonite-rich, and greisen-like melts at 820oC and 1 kbar differ insignificantly from each other and make up =0.18, 0.13, 0.14 and 0.10 wt% Ta, respectively. These values are approximately by one order of magnitude higher than mean contents of tantalum in ores of the deposit and are practically coincident with its maximal contents which does not contradict the possibility of magmatogenic concentration of tantalum in ores.
References:
#This work was supported by the RFBR (grant N 99-05-64106 and grant leading research schools 96-15-98333).
46
Fig.1. Dependence of the tantalum contents on manganese in the melts of Li-F granite (gr), albite-rich (ab), amazonite-rich (kf), and greisen-like (gs) compositions
Fig.2. The effect of temperature on the solubility products (Ksp) of MnTa2O6 in granitic liquids at 2 kbar by Linnen and Keppler (1997). ANK refers to the molar Al/(Na+K) of the melt. Our data of (Mn,Fe)(Nb,Ta)2O6 are given as mean values for the melts of Li-F granite (gr), albite-rich (ab), amazonite-rich (kf) compositions at 820oC and 1 kbar.
Fig.3. The effect of melt composition on the solubility products (Ksp) of MnTa2O6 in granitic liquids at 800oC and 2 kbar by Linnen and Keppler (1997). Our data of (Mn,Fe)(Nb,Ta)2O6 are given as mean values for the melts of massive Li-F granite and the individual layers of banded granitic rocks. at 820oC and 1 kbar (abbreviations see Fig.2.).
#Shmonov V.M., Vitovtova V.M., and Zharikov A.V. Experimental study of the effect of seismic oscillations on rock permeability at high temperatures and pressures.
Designing of underground radioactive waste (RAW) depositories has to take into account "safety reserve" i.e. it has to take into account most unfavourable scenarios of the events evolution which can lead to RAW contamination of the biosphere. One of such events can be earthquakes.
A specially designed experimental apparatus was used to determine permeability with a simultaneous attack onto a sample with seismic oscillations, differential stress, and heating (fig.1).
Fig.1.Example of experimental cycle for one of the samples: simultaneous recording of the confining pressure and pore pressure. Interval 1 - exposing at initial run parameters. Interval 2 - cyclic pressure change. Interval 3 - return to initial run parameters. Interval 4 - decrease of the pore pressure as a result of the commenced spontaneous fluid filtration through the sample.
The seismic attack was modelled by a change in the confining pressure. The permeability measurements were carried out gabbro, basalt, and limestone samples. The confining pressure was 23-25 MPa, the oscillating pressure amplitude was 0.5, 8 and 9 MPa, the frequency was 0.065 and 0.33 Hz.
#The work has been sponsored by the RFBR (Grant N 95-05-15354).
47
The pore fluid (water) pressure ranged from 4 to 20 MPa. The temperature ranged from 20 to 250oC. The oscillating pressure attack time period was 1/4 to 55 h. The experimental results show that the seismic attack affects the rock permeability and can lead to its decrease or increase. The effect depends on the oscillation amplitude, effective pressure and experimental temperature as well as on physicochemical properties of rocks.
It has been shown experimentally that seismic waves are capable of increasing the rock permeability, in particular, of limestone, by a factor 0f 1.2 at weak earthquakes (magnitude M3) at a distance to 0.67-1.75 km, at moderate earthquakes (M5) - to 32-100 km, at M7 - to 1000-3000 km from the epicentre. Heating enhances this effect - at T=250oC the permeability of basalts increased by 2.5-3.7 times after the analogous action.
For detailes see in [1].
References:
#Shmonov V.M., Vitovtova V.M., Graphchikov A.A., Kotel'nikov A.R., Sretenskaya N.G. Relationship between rocks electric conduction and permeability under the continental crust conditions.
Magnetotelluric probing (MTP) is a direct method to study in situ the present-day structure and fluid regime of the earth crust. As suggested by MTP data the continental crust is bilayered: the electric conductivity of the upper layer can be no less than 10-5 ohm-1m-1 which attests its extremely low permeability, and the electric conductivity of the middle crust is several orders of magnitude higher.
We have performed a numerical estimation of electric conductivity alteration in rocks at a change in their permeability. The calculations are based on experimental permeability data in amphibolites, gneisses, and granodiorite obtained in the temperature range 20-600oC and effective pressure 50 MPa. 0.1 M NaCl and 3.3 M KCl solutions were the conducting media. It has been found that for regions with low (9 oC/km) and mean (15 oC/km) heat fluxes an increase in temperature and pressure (depth) has to result in a monotonous decrease of the electric conductivity (fig.1). For regions with a high heat flux (26 oC/km) at a temperature in excess of 500oC and depth more than 20 km the electric conductivity can grow in a jumpwise manner. Illustrated in fig.1 are the calculation results for 0.1 M NaCl solution. With the fluid composition equivalent to 3.3 M KCl the electric conductivity values reach the number order characteristic of the middle and lower crust of the Cenozoic acitivization zones.
The obtained data correlate with the MTP ones and are indicative of a possible contribution of the temperature trend of rocks permeability to their electric conductivity values.
For details see [1].
References:
Fig.1. Calculated values of electric conductivities in rocks with low (a), mean (b), and high (c) thermal fluxes. The electric conductivity scale ( r) is given in logarithmic scale. Symbols p and n at sample numbers signify that permeability data parallel with and perpendicular to the layering direction were used in the calculations.
r) is given in logarithmic scale. Symbols p and n at sample numbers signify that permeability data parallel with and perpendicular to the layering direction were used in the calculations.
##Shmonov V.M., Zaraisky G.P., Vitovtova V.M., Matveeva S.S., Pavlova T.G. Evolution of the pore-crack space and a fluid flux in the region of 152 and 153 veins of the Akchatau granitic intrusion (Kazakhstan).
Rock permeability is one of the most important characteristics of physicochemical conditions of ore deposit formation significantly affecting the behaviour of ore-magmatic system evolution.
#The work has been sponsored by the RFBR (Grant N 98-05-64504).
##The work has been sponsored by the RFBR (Grant N 96-05-13354).
48
The fluid permeability of the Akchatau granitic massif has been considered as an integral characteristic consisting of pore-intergranular permeability of granite crystalline matrix, permeability of microcracks observable in tin sections, and permeability of macrocracks of the granite massif. We characterized each of the three systems and made quantitative estimations of their individual contribution to the integral fluid permeability of the massif. The permeability of the crystalline granite matrix was determined by a direct measurement in samples as a function of temperature and pressure [1], and the permeability of micro- and macrocrack systems was calculated by the known models.
As a result we give a quantitative estimation of the fluid permeability of the granite massif in its north-western part in a quartz-muscovite, quartz-topaz, and quartz-tourmaline ore greisen veins [2]. The current permeability of massifs determined from the borehole pumping data usually appears 1000 times as high as the pore permeability of massive samples of the same rocks. Other results were obtained for paleopermeability of the Akchatau massif. The likely permeability value of the Akchatau granite crystalline matrix at P-T-parameters of the greisen formation was close 10-3 mDarcy (1*10-18 m2). The crack permeability estimated for various versions of crack evolution and healing could exceed the integral massif permeability only by several times, i.e. by 7-8 times in the most active crack formation periods (fig.1.)
Fig.1.Temporal change of the effective permeability and rock permeability of the massif. a) origination of cracks and veins of various compositions, b) possible scenario of permeability alteration at series formation of 19 quartz-muscovite veins.
References:
#Bezmen N.I., Fedkin A.V., Zaraisky G.P. Experimental study of phosphorus and fluorine influence on the superliquidus differentiation of granite melts: Preliminary data .
Experiments conducted at 800oC and 2 kbar show that interactions of phosphorus and fluorine with granitic melts under H-O-C fluid presence (XH2=0.03-0.04) induce a liquid cluster differentiation of melt into peraluminous and silicic compositions. The enrichment of phosphorus, fluorine, and other volatiles in residual granitic melts might result in the cryptic and contrasting liquid differentiation, with formation of layers as a mechanism of the magma evolution and ore concentration of extremely fractionated Li-F-P granite massifs.
Introduction. The behaviour of phosphorus and fluorine in silicate melts have been the subject of intensive study in recent years in connection with a magmatic evolution of P, F-enriched rare-metal granites (London et al. 1993; Breiter et al. 1997; Webster et al. 1997; Zaraisky et al. 1997).
The mechanism of phosphorus and fluorine enrichment in granite rocks is explained by magmatic processes and/or postmagmatic metamorpfism with hydrothermal elements redistribution. However, exactly how the P,F - mineralization formed remains mostly uncertain on account of the absence of experimental data obtained at conditions of complex magmatic fluid.
The aim of this study is to assess experimentally the influence of phosphorus and fluorine on liquid evolution of granitic magma (at superliquidus conditions) under pressure of H-C-O system fluid.
#The financial supportby INTAS (Grant N 94-3188) and the RFBR (Grant 97-05-64799) is gratefully acknowledged.
49
Experimental technique. All experiments were run in the internally heated gas-media pressure vessel at 2 kbar and 800oC. The initial charges were homogeneous glasses obtained by melting rock at 1300oC and 1 atm. The starting granitic melts were powders of glasses of granite from an extremely fractionated Li-F(+P) massifs of Eastern Transbaikalia (Orlovka) and Krusne Hory / Czech Republic (Podlesi). The glasses were pressed into Pt-capsules (25 mm long, 5 mm in diameter, 0.2 mm wall thickness). In general, the starting charge contained 150 mg of glass and fluid (Table 1): water or solution of 10 wt% HF, theflon (CnF2n), P2O5, paraffin (CnH2n ), which were fed into the capsules. The Pt-capsules were sealed and inserted into the gradient-free zone of a tungsten reactor of hydrogen cell (Fig. 1). The hydrogen fugacity was controlled by an argon-hydrogen mixture in the reactor (Bezmen et al. 1992) and was 280 bar, X(H2)Ar-H = 0.1. As distinct from Ar-H2 mixtures at 800oC and 2 kbar a relation of gasses in the H-O-C system is strongly unideal. For accurate calculation of other gas fugacities we must control the oxygen fugacity or fugacity of any C-bearing gas in addition. Since the investigation is preliminary we decided not to complicate experiment and appreciate the oxygen fugacity and the molar fraction of hydrogen approximately. The calculated molar fractions of hydrogen in the capsule were X(H2)H-O-C = 0.03-0.04 without consideration of C-bearing gas solubilities in the melts. The oxygen fugacity therein was logfO2=NNO-1,5(2) that corresponds to magnetite stability. The experiments were kept 7 days, after which isobaric quenching was performed. The run products were analyzed by optical and electron microprobe (broad beam, 15-20  ).
).
Fig. 1. Reactor set up for conducting experiments under controlled hydrogen fugacity in a gas-media pressure vessel.
Experimental results. Before considering the obtained data one must note that all experiments were carried out at the temperatures exceeding of the superliquidus on at least 100-150oC. The water solubility increases in the presence fluorine (Holtz et al. 1993) and hydrogen (Bezmen et al. 1991) and the melting temperatures decrease accordingly. At XH2=0.04 the solidus curves of albite (Bezmen et al. 1998) and Qz-Ab eutectic (Bezmen et al. this volume) have a pronounced minimum with temperature depression about 30oC. It is known that CO2 and CO are poorly dissolved in the silicate melt, but the CH4 behaviour is not obvious. C-bearing gas have an essential influence on the differentiation of basic-ultrabasic melts (Bezmen et al. 1992) it is consequently the carbon was added in the experiment.
In experiments under a hydrogen-bearing fluid pressure a very low solubility of the silicate in the fluid was observed (Bezmen 1992) that gives evidence for the absence of extraction of the components from the melt by the fluid.
At the interaction of phosphorus and fluorine with Podlesi granite melt (Gr-5 and Gr-7 runs) in the presence of H-O-C system fluid in the absence of the thermal gradient and constancy of all other thermodynamic parameters, these develop cryptic layering, a gradual alteration of liquid composition along the sample height and appearance of layers ('lenses') enriched by silica (Fig. 2a, b). The upper boundary of the layers and 'lenses' is more sharp and the lower part is diffuse. One can see on the Fig.2 that in accordance with an increase of P2O5 content in the glass the Na2O concentration increase relative to K2O as well as in London et al., 1993. The increase of C-bearing gas content in the fluid phase cause accumulation of Qz-enriched melt in the upper part of sample (Fig. 2b).
Table 1. Content of the fluid components in charges
Fluids charge (mg) |
Podlesi |
Granite |
Orlovka |
Granite |
Gr-5 |
Gr-7 |
Gr-6 |
Gr-8 |
|
Teflon, CnF2n |
10 |
10 |
30 |
15 |
P2O5 |
5 |
5 |
- |
- |
Paraffin, CnH2n |
10 |
15 |
- |
- |
Water |
50 |
50 |
- |
- |
Solution of 10wt%HF |
- |
- |
70 |
70 |
F (wt%)* |
5.06 |
5.06 |
20 |
12.33 |
P2O5 (wt%)* |
3.4 |
3.4 |
- |
- |
* bulk content relative to silicate charge (150 mg)
50
The interaction of F-strongly enriched fluid with Orlovka granite melt (run of Gr-6, Table 1) results in the layering in a state of loop form extremely inhomogeneous cluster aggregates placed into silicate-enriched matrix. Three types of aggregates are distinguished (Table 2): peraluminous (17-18 wt% Al2O3) and strongly peraluminous with predominance of Na2O (26% Al2O3, 15% Na2O) and K2O (26% Al2O3, 12% K2O). Unfortunately, we can not analyze fluorine. However, according to sums the matrix is more enriched by fluid (35-40 wt% H2O +F) than inclusions (20-25 wt %). The cluster aggregates have a disarranged fibrous structure without interface, that is characteristic for solidified colloidal liquids. The decrease of F concentration (run of Gr-8, Table 1) in the run leads to the cryptic layering with formation of zones: upper, middle and lower (Fig.2c). The upper and lower zones are enriched by silica and became poor in alkalis and alumina. There are cluster aggregates and matrix in the every zone. Cluster aggregates are enriched by silica, matrix - by alkalis (Table 2).
Fig. 2. Cryptic and contrast layering of Podlesi (a,b) and Khangilay (c) granite melts obtained at its interaction with H-O-C-F-P system fluid at 800oC and 2 kbar at duration 7 days. I - zones of peraluminous granite; II - zones, layers or 'lenses' of silicic granite. a-Gr-5 run, b-Gr-7, c-Gr-8 (Table 1).
Table 2. Analytical data of the starting charges and the separated melts of cluster aggregates and matrix for Khangilay (Orlovka) granite runs (microprobe Camebax data calculated on 100 wt%).
Sample |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
SiO2 |
74.50 |
87.25 |
70.12 |
54.33 |
52.99 |
64.66 |
74.48 |
79.87 |
76.09 |
TiO2 |
0.20 |
0.12 |
0.32 |
0.17 |
0.14 |
0.27 |
0.25 |
0.19 |
0.21 |
Al2O3 |
13.80 |
6.90 |
18.91 |
26.33 |
26.53 |
13.44 |
15.18 |
13.13 |
13.54 |
FeO |
1.19 |
0.38 |
0 |
0 |
0.06 |
0 |
0.03 |
0.18 |
0 |
MnO |
0.03 |
0 |
0.15 |
0.07 |
0.16 |
0 |
0.07 |
0.23 |
0.05 |
MgO |
0.29 |
0.08 |
0.30 |
0 |
0.19 |
0.87 |
0 |
0 |
0 |
CaO |
0.29 |
0.25 |
0.30 |
0.51 |
0 |
0.09 |
0 |
0.19 |
0 |
Na2O |
3.64 |
1.59 |
4.86 |
11.95 |
4.81 |
1.93 |
3.82 |
1.6 |
3.4 |
K2O |
4.51 |
3.38 |
5.01 |
6.64 |
15.05 |
18.68 |
6.11 |
4.56 |
6.64 |
P2O5 |
0.06 |
0.05 |
0.04 |
0.01 |
0.07 |
0.06 |
0.06 |
0.05 |
0.07 |
51
1 - starting composition for experimental research (corresponds to Khangilay (Orlovka) biotite-muscovite granite; analyzed glass produced from molten whole rock pulver of sample Z-73); 2-9- experimental melt compositions obtained at interaction F-rich fluid with granite melt under pressure of H-O-C system volatiles (Gr-6: 2 - matrix; 3-5 - cluster aggregates; Gr-8: 6,7 - matrix, 8,9 - cluster aggregates).
Discussion. There are the numerous data of water, fluorine and phosphorous solubilities in silicate melts. At 800oC equilibrium between fluid and melt is attained during 3.5-4 days incidentally melt is homogeneous. At high concentration of F or P liquid immiscibility form of salt-silicate type. Salt and silicate are separated by interface. At increase of run duration homogeneity in the drops and matrix increases. Speed of quenching depending on the equipment type do not influence on crystals formation in the granite melt. In the presence of hydrogen, XH2O=0.03-0.04, silicate-silicate layering is developed. (Fig.2). The time of experiments is 7 days and the more duration of H2-enriched runs the more inhomogeneity (Bezmen 1992).
In the ours experiments obtained at superliquidus conditions the drops or inclusions with clear interface are absent. What is mechanism of the differentiation?
It is well known that silicate melts have a heterogeneous structure, i.e they are composed of silicon-oxygen complexes whose structure matches that of minerals. The fluidless silicate melts are 3-dimensional reticular polymers which are not capable of layering. The NMR, IR, and Raman spectroscopy data suggest that hydrogen interacts with the framework oxygen with formation of OH-groups and molecular water, during which the effective charge of silicon alters (Bezmen 1992). The break-down of bridge silicon-oxygen bonds gives rise to intensive depolymerization of hydrogen-bearing fluid magmatic melts.
The origin of heterogeneities is connected to the formation of fluctuating more ordered structures-clusters, which exchange particles and energy with matrix of melt. The depolymerization of melt affects the cluster formation. The degree of silicate melt depolymerization is stipulated by dissolution in the latter of volatiles, specially of hydrogen, phosphorus and fluorine: their presence increases water solubility (Surapure and Hamilton 1984; Holtz et al. 1993; Bezmen at al. 1991). Clusters are intermediate state between liquid and crystal. The popular way to describe clusters is the 'jellium' model (Cohen and Knight 1990) in which the cluster is seen as having a central ordering core of atoms surrounded by a cloud of total electrons. The structure as whole is uncharged. Moreover, clusters are put together in the associations as package, spheroids and chains which have the end molecules are capable to be exchanged with matrix (Strepiheev and Derevitzkaia 1976). Clusters are stabilized by the presence of appropriate ligands (Schmid 1985; Pruchnik 1990). Most of the pioneering experimental work has been done using complex organic reagents (see Schmid 1985), but theoretically most of the ligands (such as F, P2O5, OH) should be suitable to effect such stabilization. The ligands do not form 'formal' chemical bonds with the cluster core, but rather arrange themselves as symmetrical (usually nearly spherical) envelopes around the cluster in such a way that repulsion is minimized (Schmid 1985). The atoms in the ligand layer are highly mobile, so that the structure as a whole develops a solid-like core with a liquid-like surface (Schmid 1985). The behaviour of clusters cannot be predicted by 'classical' chemical principles: various studies on such diverse properties as ionization potentials and nearest neighbour distance has shown that the values for clusters of an element is intermediate between that obtained for its individual atoms and the bulk crystal (Stace 1988). As it was shown experimentally under certain critical thermodynamical conditions the aggregate of clusters are capable of the gravitational movement (Bezmen 1992) with the accumulation of colloidal liquids which after quenching have the disarranged fibrous solidified structures.
Experimental data shown that P and F in the presence of hydrogen and other gases of H-O-C system brings about a cryptic and contrasting layering of granite melt of silicate-silicate type (peraluminous granite-silicic granite, Fig.3). Unfortunately, we can not analyze the F distribution along height of samples, but preliminary data revealed essential increase F concentration in hydrogen-bearing silicate melts (up to 9 wt%). After crystallization owing to high F affinity to hydrogen it should be redistributed to the gas phase and abandons the silicate. We are going to verify that experimentally.
Fig. 3. The Ab-Or-Qz normative diagram of the experimental data. Solidus curves of haplogranite system in the presence of H2O (2 kb) and H2O-F fluid were constructed from experimental data given in Manning (1981) 1-starting compositions of Podlesi granite, 2-Gr-5, 3-Gr-7, 4- starting compositions of Khangilay granite, 5- Gr-6,8.
Conclusion. In the evolution of granite massifs a residual melt enriched in P,F and other volatiles. While reaching the concentration of fluid enough for a cluster differentiation this melt develop with formation of the layered rocks between which fluid and other components are distributed including of Li, Ta, Nb, and et al.
52
References:
#Balashov V.N. Chemical transport with allowance for reaction kinetics in media with variable porosity and permeability. Skarn formation induction by decarbonation - dehydration reactions in 2D rock regions.
Earlier [1,2] we proposed and developed a self-consistent model of chemical transport at rock metamorphism. The model is a principally new step in the theory of the fluid-rock interaction dynamics at high P-T-parameters. The first consideration in the model is the allowance for the diffusion-reaction feedback to the flow and rock creep intensity.
This work is a development of the two-dimensional version of the self-consistent model of chemical transport as applied to the 2D-problem [3]. We have studied the initial quasistationary stage of wollastonite formation reaction in a quartz-bearing marble stratum under the action of water-carbon dioxide filtration. The calculations were performed for the 25.25 m region with the initial homogeneous porosity and permeability. Isothermal problem was solved for 600oC and 200 MPa. Vertical filtration with the diffusion of a H2O+CO2 fluid nonequilibrium with the initial mineral association Cc+Qtz was considered. The occurrence of the wollastonite formation reaction gives rise to significant alterations of hydrodynamic fluid flow regime, fig.1. The fluid depressurisation in the reactive permeable part give rise to creep in this part followed by a partial porosity closing. As a whole a wave of increased porosity evolves throughout the marble strata, its crest being approximately oriented in parallel with the stratum and locating near the line of the full reaction completion. The porosity crest values are, for the most, close to 4%. After 200 years the crest structure has a transversal dimension from 0.5 to 3 m, fig.2. 25-30 years after the beginning of the filtration, the CO2 concentration distribution in the fluid becomes quasistationary. So, it has been quantitatively shown that decarbonation - dehydration reactions are fluid conductors of ample capacity and can, possibly, induce an intensive skarn formation process.
#The work was carried out in the University of Leeds (Great Britain), IEM RAS (Chernogolovka, Russia), and University of Oslo (Norway) and sponsored by the joint project of the Royal Society, RFBR grants 96-07-89323, 96-15-98333, and Norway Research Council grant 113354/420.
53
Fig.1. Distribution of Darcy filtration velocities by the time of 193 years. The flux focusing along the marble stratum is caused by the porosity increase due to decarbonatization reaction. The initial fluid pressure gradient was equal to the lithostatic one, the initial porosity - 1.5%. The numbers on the fluid pressure isolines correspond to difference (in MPa) between the fluid pressure and the pressure on the horizontal level with the vertical zero co-ordinate. The arrows lengths are proportional to the fluid flow rate whose maximal value is 0.13 m/y.
Fig.2. Quasistationary porosity distribution in the 2D model after 193 years.
References:
#Balashov V.N. Oscillation phenomena in zoning at magmatic crystallization with fluid escape.
The study of fluid-magmatic interaction has become of a great importance in relation to investigation of banded complex in tantalum ore deposits Orlovka and Etyka in the eastern Transbaikalia. This complex includes layers of quartz-albite, quartz-K-feldspar (amazonite) and quartz-mica ('greisen') composition and occurs in the domes of Li-F granite stocks. A model of crystallization from quasi-two-component melt, with account of interaction with fluid (third) component in combination with nonequilibrium process of fluid accumulation/escape, has been proposed. At specific regime of fluid component accumulation in melt, the latter falls in highly nonequilibrium area. Theoretical calculations show that in this case the oscillation behavior of fluid escape from the melt may occur.
A simplified system albite K-feldspar at Ptotal = PH2O+HFfluid = 100 MPa is used as the basis for the model. The main simplification is in the consideration of the system without formation of a solid solution. The shift of eutectics under influence of fluid fluorine-component was calculated on the basis of data from [1].
A kinetic scheme of proposed model is written as follows:
#The work has been sponsored by the RFBR (Grants N 96-07-89323, 96-15-98333).
54
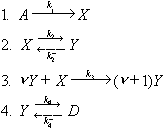
In the proposed scheme reaction 1 corresponds to external gain of fluid component in passive form, while reaction 4 complies with the exchange of component in active form with fluid phase D. In essence, the scheme at n=2 is very close to chemical oscillating 'brusselator' system [2]. The performed investigation reveals a wide area of oscillation phenomena in parametric space k1 k3, where k3 is a constant of autocatalysis and k1 is a constant of fluid component addition. The path of crystallization in the system and relative enrichment of crystallizing layers in albite component with time at k1 = 0.86.10-6 s-1 and k3 = 0.86.10-4 s-1 are shown in Fig.1,2. Initial renormalized K-feldspar mole fraction and melt temperature were 0.35 and 750oC, respectively. The constant of autocatalysis exerts the most sufficient influence on oscillating characteristics of the process. With increasing this constant, the stationary temperature of crystallization also increases. In the above example, this value is 717.5oC and oscillations come around it (Fig.1). An increase in the rate of fluid component addition intensifies the oscillation phenomena, beginning in the end of crystallization or, spatially, in the central part of crystallizing layer (Fig.2). In the phase area, paths of crystallization are attracted by a line of eutectics of the different fluid composition liquidi.
The slowing down of a cooling rate does not noticeably change the time frequency of oscillations but increases the spatial density of oscillations, thus favoring the possibility of development of the small-scale periodic structures in the limits of individual grains. The oscillation process, at decreasing of cooling rate, emphasizes the liquidus of specific composition and corresponding eutectics.
Fig1. The path of albite-K-feldspar melt crystallization at interaction with fluid component. The mole fraction of K-feldspar recalculated on dry melt is plotted on the X-coordinate axis. Liquidus lines correspond to mole fraction of fluid component in the melt 0.325. Comments in the text.
Fig.2. Distribution of relative enrichment in albite in crystallizing layers with time. The time unit on the X-axis is equal to 6.39 years. Comments in the text.
References:
#Balashov V.N. Fluid flow and irreversible compaction of rocks in sedimentary basins.
The theoretical quantitative investigation of pore solution flows at gravitational compaction of sedimentary rocks is performed. This theme is a part of the strategic program-project 'Fluid-rock interaction' of the Department of Geology, University of Oslo, Norway. Professor Bjorn Jamtveit is a supervisor of the program.
The process of irreversible compaction of sedimentary rocks by the mechanism of 'solubility under pressure' is examined in sequence by the two-dimensional mathematical simulation. The experimental and theoretical data on the irreversible deformation of polycrystalline quartz aggregate by the mechanism of pressure solution reveal high rates of the process under conditions of sedimentary basins. Then, the compaction of 2-3 times may be expected for the period of n tens of thousand years, where n = 2-9. However, geological data testify about periods in n tens of million years, thus, a discrepancy of 2-3 orders is evident. One of the possible explanation of this disagreement is a suggestion that pressure solution is sufficiently slowed down under the real natural conditions.
#This work was supported by a grant 113354/420 of Norwegian Research Council.
55
We have shown another possible way to resolve this contradiction [1,2]. In brief, the essence of a phenomenon is an existence of the critical ratios of the parameters permeability and effective viscosity of the porous medium. It is important for the typical model of sedimentary basin with impermeable layer (Fig.1) when 'entrapment' of porosity occurs under impermeable layer and flow of expulsed solutions is maintained for a long time (10 millions years). The permeability of medium approaches in this case the values typical of sedimentary shale (lower than 10-17 m2). Porosity distribution acquires a highly heterogeneous character (Fig.2) with formation of overpressured reservoir in the zone adjacent to impermeable layer. Spatial dimensions of the model correspond to 5 km depth and 15 km horizontal direction.
Hence, maximally approaching the geological nature, it is necessary to take into account the physico-chemical processes, leading to irreversible deformation slowing down, as well as structural peculiarities of the macroprocess of sedimentary rock compaction with possible generation of high pressure porous reservoirs.
Fig.1. Porosity distribution for the two-dimensional model area at initial permeability of medium 8.10-18 m2 and initial porosity 20% for the period of 1078 years.
56
Fig.2. Porosity distribution for the model Fig.1 at 11.1 million years.
References:
57