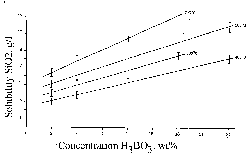
IX. Synthesis and modification of minerals
(Leader Prof. V.S.Balitsky)
#Balitsky V.S., Balitskaya L.V., and Mar'ina E.A. Solubility and growth of quartz crystals in water-borate solutions.
Solubility of quartz was studied, and its single crystals were grown in solutions of boric acid with concentrations from 3 to 24 wt.% at temperatures 400, 500, 600, and 700oC and pressure close to 1.4 kbar (the pressure was estimated from the P-V-T diagram for neat water). The results of studying the quartz solubility are shown in the plot and indicate that the presence of and increase in the concentration of H3BO3 in solutions as well as the temperature increase enhance substantially the quartz solubility. Under conditions of the direct temperature gradient (20-80oC by external thermocouples), silica is intensely transferred and quartz crystals grow on seeding in the upper (relatively less hot) zone of the autoclave. The role of borate ions in the silica transfer has not been discussed previously and was not taken into account in studying conditions of formation of borosilicate minerals.
The experimental data obtained make it possible to understand and explain the formation of large deposits of borosilicate minerals, datolite and danburite, in skarned limestones.
Fig.1. Isotherms of quartz solubility in aqueous solutions of boric acid.
#Balitsky V.S. and Balitskaya L.V. Experimental modelling of simultaneous processes of dissolvation and growth of quartz and topaz crystals.
Experimental modelling of simultaneous processes of dissolvation and growth of quartz and topaz crystals in supercritical acidic water-fluoride fluids under conditions of direct temperature gradient from 500 to 900oC and pressures from 0.2 to 5 kbar was performed. The scheme of experiments is shown in the figure. The most important result of the studies is the experimental evidence that zones of dissolvation and crystallization of topaz and quartz can either coincide or be spatially separated. In low-dense fluids (< 0.33 g/cm3), quartz (both individual and in the presence of topaz) is dissolved in a relatively lower-temperature zone and transferred to a higher-temperature zone, i.e., its transfer is opposite to the T vector.
In denser fluids, the directions of silica transfer and direct thermogradient coincide as usual. In similar fluids, topaz behaves in a different way. In the absence of quartz, it is virtually insoluble and is not transferred even at 600-800oC and pressures to 1.6 kbar. However, in the presence of quartz, intensities of dissolvation and transfer of both minerals become comparable. Unlike quartz, topaz crystals both in low-dense and dense acidic water-fluoride fluids always dissolve in a less high-temperature zone and always grow in a more high-temperature zone.
The experimental data obtained made it possible to prove unambiguously for the first time that spatially conjugated and spatially separated processes of dissolvation and growth of quartz and topaz crystals can occur simultaneously under thermogradient conditions. Under natural conditions, similar phenomena are observed in chamber pegmatities and greisens [1]. As follows from the experiments, the composition, pH, and density (pressure) of supercritical fluids have the basic effect on the spatial conjunction and separation of dissolvation and crystallization zones of these minerals.
# This work was supported by the Russian Foundation for Basic Research (project no. 97-05-64805).
56
Reference:
Balitsky V.S. Growth conditions and properties of mineral crystals.
Experimental study has been carried out on the processes of the separate and joint dissolution and crystallization of quartz, corundum, topaz, and tourmaline in supercritical water fluids. The experiments were run at T=500-800oC and P=0.4-1.5 kb in pure water and aqueous solutions of hydrofluoric and boric acid, NaOH and NaCl.
It was found that at given parameters tourmaline in aqueous solutions of H3BO3 of the concentration less than 6% is extremely inert even at the gradient up to 100oC (external measurement). With increasing H3BO3 concentration up to 12 wt% both seed and charge tourmaline crystals get covered with (substituted by) thin schorl film. Moreover, well edged small (parts of mm) schorl crystals form on the surface of the seed crystal. Charge crystal shows poor dissolution. The presence of quartz causes no essential changes in the character of tourmaline dissolution and transfer. The quartz itself in the presence of tourmaline dissolves intensively and transfers
57
from the zone of high temperatures to that of lower temperatures . In fluoride solutions tourmaline is unstable, dissolves intensively to form at least two fine crystalline phases (under determination) which cover both seed and charge crystals . The presence of quartz in the system does not change the character of dissolution of minerals.
The intensity of silica transfer in borate solutions (with H3BO3 concentration of 6 and 12 wt%) at T=500, 600, 700, and 750oC is much higher than the intensity of its transfer in pure water and chloride aqueous fluids, but is less than in alkaline aqueous fluids and is comparable with the transfer in fluoride acid fluids . These results were used for the experiments on seed stimulated growing of quartz crystals in borate aqueous fluids. The crystals grown, colourless and of primary pink violet coloration, were 20-30g in weight.
Novikov G.V., Sipavina L.V., and Sokolov Yu.A. Local fields and structure features of two Fe-Ca monoclinic chain germanates.
Chain germanates along the join (Ca,Fe)GeO3 with the pyroxene structure were studied by means of 57Fe gamma-resonance and X-ray powder diffraction methods. Depending on the temperature and on the Fe-to-Ca ratio two distinct monoclinic structures with the same space group C2/c were determined. The transition from the low-Ca to the high-Ca structure with the volume jump of 4.4 % is accompanied by the change in local field parameters at the 57Fe nuclei both in M1 and M2 crystallographic positions. As it was shown by 57Fe gamma-resonance in the wide range of temperatures, the electronic state of Fe2+ cations in M2 crystallographic positions is very close in these germanates to that in M2 sites of relative pyroxenes, being identical to state of Mg-bearing orthorhombic pyroxenes, for the low-Ca Ge pyroxenes, and, for the high-Ca Ge pyroxenes, to the state of Fe2+ cations in M2 sites in the high pressure monoclinic ferrosilite structures (both with P21/c and C2/c s.g.) as well as in orthoferrosilite.
The most important results were found for M1 positions in germanates studied. As it was determined at low temperatures, the M1 sites in the high-Ca structure are able to contain the Fe2+ ions with two distinct electronic structures, giving two distinguishable 57Fe doublets (M1a and M1, Fig.1).
Fig. 1. The N-transformed 57fFe spectrum of Ca-rich Ge- pyroxene at low temperature.
Earlier attempts to interpret the low temperature patterns of Fe2+ in the relative Ca-bearing pyroxenes were not successful because of poor resolution of their 57Fe spectra. We solved the problem of decomposing of complex spectra of Ge pyroxenes using the original mathematical procedure. For all we know, it is the first direct determination of distinct electronic states of Fe2+ in the formally equivalent crystallographic positions M1 in the chain structures.
In the low-Ca structure the electronic state of all Fe ions in M1 positions is identical, giving the hyperfine parameters, which are close to ones of the 'M1a' state of Fe2+ ions in the Ca-rich structure. Hyperfine parameters of Fe2+ ions in the 'M1' state in the Ca-rich structure are very close to these parameters in hedenbergite.
The space group C2/c of germanates under investigation was supported by the qualitative inspection of relative intensities in their X-ray powder diffraction patterns. Coordinates of cations were estimated from these intensities also, using original method, based on the least squires principle. Oxygen coordinates were proposed being the same, as in two relative structures - MgGeO3 and CaFeSi2 O6. Polyhedra M1 and M2 in the low-Ca structure have the geometries, similar to ones in pyroxenes with the kinked SiO4 chains, and in the high-Ca structure - in pyroxenes with the straight chains.
Novikov G.V., Sipavina L.V., and Konilov A.N. Fe electron state features in hemoilmenites.
The anomalous behavior of physical properties of hemoilmenites (1-x)FeTiO3 x Fe2 O3 due to the specific features of the iron electronic state at 0<x<0.5 were determined long ago from many physical measurements, but their nature was not clear so far. In our study the electronic state of iron ions in these solid solutions was shown being changed at x = 0.03. Two distinct types of the charge and spin states of iron ions in hemoilmenites along this join were found and studied by 57Fe gamma-resonance, using textured samples and external magnetic fields.
Unusual reaction of Fe2+ electronic state in the paramagnetic FeTiO3 was found in external magnetic field of 12 kOe: - two times more stronger hyperfine magnetic field of 23 kOe was recorded on the 57Fe nuclei. Magnetic pattern in the spectrum of powder sample consisted of 8 distinct components (Fig. 1, T = 300K).
Fig. 1. The N-transformed 57Fe spectrum of ilmenite in the external field ( H = 12 kOe). Solid line - calculated theoretical spectrum. The corresponding HFS parameters are presented on the Fig.
58
At the same time the other, potentially ferrimagnetic hemoilmenites (0.04<x<0.20, T = 300K) in the external magnetic field of 12 kOe have shown the predictable magnetic pattern, typical for paramagnetic powder sample in the magnetic field. It was one of distinctions between two different forms of electronic state of iron ions in hemoilmenites.
There is one more distinction between this unusual, but 'clear' electronic state of Fe2+ ions in the end member FeTiO3 and iron state in the solid solution at 0.04<x<0.20. The low temperature 57Fe spectra of solid solution contained both the pattern, corresponding to the electronic state of Fe2+ ions, identical to one in the end member FeTiO3 , and some additional pattern, which has to be refered to other kind of electronic state(s). The partial intensity of the 'clear' Fe2+ magnetic pattern decreases up to 3 times when temperature decreases. To interpret these experimental data the dynamic charge state of iron ions and the existence of Ti3+ ions were proposed in hemoilmenite solid solution at 0.04<x<0.20 at low temperatures.
In general, the two-doublet model, accepted in literature for ilmenite-rich part of the join, in real does not fit the 57Fe spectra of paramagnetic hemoilmenites. The additional to the pure Fe2+ state other charge and probably spin states of iron are to be assumed. According to our results, the local method of the 57Fe gamma-resonance spectroscopy and the traditional bulk magnetic susceptibility measurements give the contradictory statements on the magnetic ordering temperature in the hemoilmenite solid solution.
Romanenko I.M. Cheralite from visakhapatnam area in the eastern ghats granulite belt.
Determination of a cheralite composition by EPMA. The main problem in electron probe microanalysis (EPMA) of rare earth elements (REE) minerals is connected with x-ray lines interferences and measuring of background intensity. The following technique of wave dispersive EPMA REE minerals is offered in [1]:
1) measuring of characteristic intensity analytical x-ray line (for example, LaL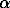 -line) on reference sample LaPO;
-line) on reference sample LaPO;
2) measuring of intensity interferences lines for LaL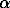 -line (lines of Ce and Nd) on reference samples CePO and NdPO;
-line (lines of Ce and Nd) on reference samples CePO and NdPO;
3) measuring of background on background sample for LaL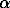 (this is PrPO in table 1 );
(this is PrPO in table 1 );
4) calculation of net intensity of LaL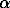 -line with subtract of interferences lines and background intensity.
-line with subtract of interferences lines and background intensity.
It is very important to hold order of measuring analytical line. The calculation of interferences and background intensity is carried out taking into account the differences of effective atomic numbers of reference and unknown samples.
WD EPMA of a cheralite composition was carried out on CAMEBAX-MBX with energy dispersive x-ray spectrometer Link-860/500 and two wave dispersive spectrometers.
As wave dispersive (WD), so energy dispersive (ED) EPMA electron beam energy was set 20 keV. Current of beam was 30 nA (under wave dispersive) and 1 nA (under energy dispersive).
Energy dispersive EPMA data are not so comprehensive as wave dispersive, but they can be used for the preliminary study of unknown materials. The measuring time of reference REE spectra is equal to 500 s, and of unknown spectra - 200 s. The samples are natural and inhomogeneous.
All data for age determination (the concentrations of Pb, U and Th) were obtained by wave dispersive spectrometers.
References:
#
Stolyarova T.A. Chemical composition and thermodynamic properties of apatite minerals.Generalization of data on natural apatites has made it possible to reveal isomorphic mineral series (fig.1) for a thermochemical study.
Fig.1. Position of the thermochemically analyzed apatites on the diagram with respect to theoretical compositions of apatite (Ap) and whitlokite (Wh). 1- theoretical compositions; 2-compositions of the analyzed apatites (from table 1).
# The work was supported by the RFBR, Project N97-05-64159.
59
An examination of fluoroapatites containing small concentrations of chlorine and water typical of alkali nepheline syenite intrusives from the Kola peninsula (Khibinsky, Lovozersky, and other massifs) exhibits a large deficiency of fluorine with respect to the theoretical composition of fluoroapatite (3.8 wt% F) Fluoroapatite is found to be almost completely isomorphic with calcium phosphate that is free of volatiles (compositionally close to whitlokite).
Samples with variable fluorine concentrations were used in a thermodynamic study of this isomorphic series. The purity of the examined material was carefully controlled under a microscope. A chemical analysis of the minerals was performed in the chemical laboratories of IEM RAS and NP SB RAS. The concentration of fluorine was also analyzed by a channel microanalyser technique (table 1).
The determinations of the dissolution enthalpy of natural and synthetic apatites were performed on a differential automated microcalorimeter DAK-1A produced at the Experimental Plant for Scientific Instrument Engineering, RAS, in Chernogolovka and modified by us for work in aggressive media.
The dissolution conditions for samples were selected in preliminary runs: the concentration of the aqueous solution of hydrochloric acid 20% wt, the solvent volume 3.5 ml, the charge 1-3 mg, the dissolution temperature - 40oC. A 'Sartorius' balance was used for weighing, the precision was 10-6. The cells were electrically calibrated, the calibration being checked before and after each measurement. A least squares technique was used to process the results of the determinations.
The results of the experimental measurements of formation enthalpies of apatites are listed in table 2. The apatite formulae calculated from the analyses are given in the form of solid solutions of apatite-whitlokite pairs (1-n) wh. Sample 5-7 were examined in the current year. The data complemented the scenario of the behavior of the fluoroapatite-whitlokite series: a significant reduction of the stability region of fluoroapatite.
With a decreased fluorine concentration in it against the theoretical stoichiometric composition that is almost absent in natural mineral formation.
Table 1. Compositions (wt%) examined thermochemically.
|
Components |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
|
SiO2 |
0.70 |
0.46 |
- |
0.21 |
0.64 |
0.43 |
0.44 |
1.02 |
1.01 |
|
Al2O3 |
- |
0 .02 |
0 .02 |
0 .02 |
0 .01 |
0 .02 |
0 .08 |
- |
0 .03 |
|
Fe2O3 |
- |
0 .22 |
0 .53 |
0 .04 |
0 .61 |
0 .45 |
0 .13 |
0 .05 |
0 .06 |
|
La2O3 |
- |
- |
- |
0 .35 |
- |
- |
- |
- |
- |
|
Y2O3 |
- |
- |
- |
0 .04 |
0 .15 |
- |
0 .05 |
0 .12 |
0 .17 |
|
MgO |
- |
0 .05 |
0 .01 |
- |
- |
0 .02 |
0 .15 |
0 .09 |
0 .06 |
|
MnO |
- |
0 .03 |
- |
- |
- |
- |
0 .05 |
0 .02 |
0 .02 |
|
CaO |
56 .20 |
55 .09 |
54 .72 |
53 .46 |
56 .06 |
55 .22 |
52 .66 |
53 .43 |
54 .64 |
|
SrO |
- |
0 .13 |
1 .13 |
3 .15 |
- |
- |
3 .78 |
3 .73 |
2 .13 |
|
K2O |
0 .04 |
- |
0 .06 |
0 .11 |
0 .09 |
0 .06 |
0 .13 |
- |
- |
|
Na2O |
- |
- |
0 .28 |
0 .13 |
0 .17 |
0 .41 |
0 .22 |
- |
- |
|
P2O5 |
40 .00 |
40 .35 |
41 .63 |
39 .72 |
39 .37 |
42 .00 |
40 .63 |
40 .42 |
40 .67 |
|
H2O |
0 .33 |
0 .03 |
- |
0 .18 |
- |
- |
0 .40 |
0 .20 |
0 .22 |
|
CO2 |
- - |
1 .10 |
- |
- |
0 .30 |
- |
1.00 |
0.29 |
- |
F |
2 .80 |
3 .29 |
3 .00 |
2 .00 |
2 .43 |
2 .24 |
1 .17 |
0 .90 |
1 .06 |
Cl |
- |
0 .03 |
0 .40 |
0 .15 |
- |
- |
- |
- |
|
Sum |
100 .07 |
100 .88 |
101 .38 |
99 .81 |
99 .98 |
100 .82 |
100 .92 |
100 .27 |
100 .07 |
Note: Apatite samples from the Khibinsky massif (1,2,4,6,7,), Kovdor (8,9) and Slyudyanka
Table 2. Formulae and thermodynamic properties (in kcal) of the examined natural apatites (1-9), synthetic apatites (10-12), and whitlokite (13)
| N | Formulae of apatites | - Hdiss298.15 Hdiss298.15 | - Ho298 Ho298 | - Go298 Go298 | S. cal |
| 1 | 0.910 Ap. 0.090 Wh | 18.17+0.33 | 1603.00 | 1532.89 | 94.010 |
| 2 | 0.932 Ap. 0.068 Wh | 22.79+0.26 | 1590.82 | 1510.56 | 92.830 |
| 3 | 0.775 Ap. 0.225 Wh | 21.74+0.66 | 1575.76 | 1496.56 | 93.135 |
| 4 | 0.727 Ap. 0.273Wh | 18.51+0.20 | 1625.90 | 1545.10 | 94.190 |
| 5 | 0.685 Ap. 0.315 Wh | 26.27+0.21 | 1600.61 | 1505.70 | 93.462 |
| 6 | 0.577 Ap. 0.423 Wh | 22.47+0.48 | 1585.90 | 1506.36 | 91.315 |
| 7 | 0.581 Ap. 0.419 Wh | 18.25+0.18 | 1566.10 | 1483.40 | 92.830 |
| 8 | 0.249 Ap. 0.751 Wh | 30.77+0.55 | 1504.78 | 1424.62 | 92.041 |
| 9 | 0.250 Ap. 0.750 Wh | 31.49+0.60 | 1512.48 | 1353.23 | 88.480 |
| 10 | Ca5P3O12OH | 34.30+0.53 | 1609.15 | 1515.06 | 93.188 |
| 11 | Ca5P3O12F | 15.80+0.30 | 1643.42 | 1563.57 | 95.700 |
| 12 | Ca5P3O12Cl | 29.05+0.36 | 1584.72 | 1498.99 | 99.300 |
| 13 | Ca4.5P3O12 | 37.30+0.5 | 1473.44 | 1393.39 | 86.850 |
60
#Chichagov A.V., Varlamov D.A., Dilanyan R.A., Dokina T.N., Drozhzhina N.A., Samokhvalova O.L., Ushakovskaya T.V. The local and www-adapted versions of the software system on crystal structures of minerals (MINCRYST-97).
MINCRYST is the original consistent combination of the database on Crystal Structures of Minerals, Subordinate database on Calculated Polycrystalline Standards, and Applied Software Package [1, 2].
The local version of the MINCRYST is designed for operation at an individual PC or PC within a local network (PC Pentium, Windows 95/3.11, database Borland DELPHI 2.0, format - Paradox 5.0).
The adaptation of the DOS-operating Applied Software Package for Windows is the final step in the development of the local MINCRYST version.
To make MINCRYST available for all interested users, the development of the web-adapted version of the system was started in 1997. Access to the MINCRYST database through Internet was arranged with the help of new network technologies [3]:
Browser <--> Internet <--> HTTP server <--> request menu <--> database in SQL format
The following software and hardware facilities were used: DEC AlphaLX-533 workstation, OC Digital Unix, HTTP Apache server, JavaScript and PHP/FI languages, and mySQL database server.
MINCRYST is presently available to all interested users at the website http://database.iem.ac.ru/mincryst/.
The developed version of the www-interface, which employs the tools of the HTML-Language for request formation, transforms a simple user' s request into a complex structured request to the MINCRYST database. This program allows a user to browse, search, and select records by a number of parameters (mineral name, chemical composition, unit cell parameters, spatial group, interplanar space, references, etc). The complete answer to user's request is formed as an Information Chart, which shows structural characteristics of a mineral as a single crystal and as a polycrystalline standard.
The organization in Internet of interactive work with the Applied Software Package also seems very promising.
The development and www-adaptation of several programs producing images of crystal structures of minerals, calculating lengths and angles of chemical bonds, and modeling X-ray diffraction patterns of minerals and mineral mixtures are now underway.
The www-adapted version of MINCRYST is now a constituent of the www-server of the Institute of Experimental Mineralogy (IEM RAS).
References:
Romanenko I.M. EPMA of background components.
The background components of X-ray spectrum induced by electrons under the conditions of EPMA were studied using microprobe JXA-5A (JEOL) and spectrum analyzer NTA-512M (Hungary). Two main components of the background were found: (1) the bremsstrahlung emission of target cut by crystall analyzer of WDS and (2) the emission induced by backscettered electrons on a parts of spectrometer and window of gas-flow proportional counter. The summary intensity of background is represented as:
Nb = kbrZeff(Eo- Eq)/Eq + ks( Eo)5, 1)
where kand k are constants; E and E are electron beam and X-ray line energies; Z = 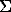 is effective atomic number of target.
is effective atomic number of target.
The contribution of second member in summary intensity of the background fast rises from the electron beam energy of 15 keV. Experimental and calculated data agree with each other from 5 to 45 keV energies of the electron beam.
Unusual high displacement of average peak amplitude on output of preamplifier was found under low pulse intensity (from 1 to 300 ps/s) was found. The way of the upper level set of amplitude analyzer in differential mode is suggested. In an ordinary set of the upper level some part of pulses will disappear (to 20%). The upper level can be calculated by the equation:
V = Vust(1 + 0.02Eq), (2)
where E is X-ray line energy; upper level, V is a high level of the analyzer window set on a sample with high concentration of determination element (more than 10 wt.%).
# MINCRYST development is supported by the Russian Foundation for Basic Research, projects nos. 96-07-89162 and 96-07-89323.
61