VIII. Disposal of high level wastes
Suvorova V.A., Zyryanov V.N., Kotelnikov A.R. Synthesis and properties of ceramic matrixes for the fixation of radioactive iodine.
The objective of this work was to investigate absorption of iodine by different zeolites with their following ceramization. The technique employed was similar to that described in [1] concerning the Sr-containing zeolite ceramization. Only stable iodine isotope was used in the experiments. Zeolites of two types were used: (1) industrially prepared synthetic NaX-zeolite of the composition Na2O.Al2O3.2.5SiO2.6H2O was chosen for its high (63%) sorption capacity to iodine [2] and capability of yielding ceramics with sodalite structure; (2) industrially prepared Cu-zeolite (IONEX) obtained by substitution of Na-ion for Cu-ion in the standard NaX zeolite structure was chosen for its low price, compared with Ag-zeolite, while CuI and AgI are similarly stable (log(KdissCuI) = -11.96, log(KdissAgI) = -12.4). The completely saturated Ag-zeolite is known to absorb up to 70% of iodine from the gaseous streams produced in cutting fuel elements. Ag-zeolite can absorb up to 250 mg of iodine per 1 cm3 [2].
Experimental and analytical methods
The initial zeolites were preliminarily dried at 110oC and annealed at 300oC during 24 h for dehydration to attain constant weight. Iodine was introduced in different ways. The dehydrated zeolite samples together with the excess of crystalline iodine were held in evacuated glass capsules at 130-400oC during 12-24 h. Another method of introduction of iodine into zeolite consisted in the synthesis of ceramics from the dehydrated initial zeolite in the presence of iodine excess and water (1 wt %) in hermetically sealed platinum capsules at 500-800oC and 100 MPa. Quenched samples appeared as dense cylinders 30 mm long and 3-5 mm in diameter. Iodine was also introduced into NaX zeolite through the ceramiza-
51
tion of the zeolite together with dry CuI (20 wt %). In some runs introduction of iodine into the initial Cu-zeolite was preceded by copper reduction. The copper reduction experiments were carried out in sealed quartz tubes divided in two by a detachable nickel gauze. Pure graphite was put on the bottom of the tube, and the granules of dehydrated zeolite were placed on the gauze. The temperatures on the levels of graphite and zeolite were 750-780oC and 280-500oC, respectively. Copper reduction was due to the interaction of copper oxides and carbonates with CO produced as a result of heating graphite above 700oC. Reduced and iodine-saturated zeolites were dried at 100oC during 2 days and then subjected to ceramization in gasostat at 800oC and 100 MPa.
Table . Conditions and experimental results of zeolite ceramization.
|
N N runs |
Weigth of initial zeolite, g |
Addition I2, wt % |
Addition CuI, wt % |
T, o C |
t, days |
d, g/ sm3 |
P, % |
X-Ray diffraction data |
|
8 |
0.264 |
- |
- |
500 |
4 |
2.34 |
8.2 |
Ab1) |
|
10 |
0.326 |
20 |
- |
-'- |
5 |
- |
- |
Ab + NaI + I-sodalite |
|
23 |
0.400 |
7 |
- |
-'- |
3 |
2.42 |
3.3 |
-'- |
|
24 |
0.600 |
20 |
- |
-'- |
-'- |
2.45 |
1.4 |
-'- |
|
13 |
0.519 |
- |
20 |
-'- |
6 |
2.41 |
0.2 |
Ab + CuO+ CM2) |
|
19 |
0.904 |
- |
21 |
1000 |
3 |
2.48 |
0.0 |
Ab +CuI +CM |
|
21 |
0.747 |
- |
20 |
400 |
3 |
2.51 |
1.1 |
-'- |
|
7 |
0.491 |
- |
- |
500 |
9 |
2.45 |
10 |
CM |
|
15 |
1.651 |
20 |
- |
-'- |
6 |
1.68 |
7.2 |
CM + CuI |
|
20 |
1.114 |
41 |
- |
-'- |
3 |
2.55 |
3.1 |
-'- |
|
27 |
0.645 |
10 |
- |
-'- |
-'- |
2.74 |
0.0 |
CM + weak CuI |
|
28 |
0.813 |
5 |
- |
-'- |
-'- |
2.53 |
0.3 |
-'- |
|
29 |
0.776 |
~30 |
- |
800 |
1 |
2.49 |
18 |
CM + CuI |
|
31 |
0.548 |
-'- |
- |
-'- |
-'- |
2.34 |
22 |
-'- |
1) - Albite
2) - ceramic matrix (CM) based on Cu-zeolite (mixture of quartz, corundum and copper oxides)
Complex thermal analysis of the initial zeolite samples was performed with Q-1500D derivatograph, and infrared spectra of the samples were obtained using Perkin-Elmer 983 spectrophotometer. Density, d, and open porosity, P, of the samples were determined to evaluate the ceramics quality. The results are given in Table. The X-ray patterns obtained were decoded using ASTM card catalog. The X-ray patterns of products of NaX zeolite ceramization exhibit weak NaI reflexes, the phase of albite type formed by ceramization of the initial zeolite without iodine, and the new phase of composition corresponding to iodosodalite synthesized at 700oC and 0.1 MPa [3]. The compositions of run products were analyzed by Camebax microprobe with Link detector with accuracy 1-3 rel. % regarding the main elements.

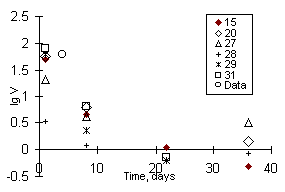
Fig.1. Leaching rate of iodine from ceramic samples based on Cu-exchanged zeolite; in legend indicated numbers of runs. Data - Shidlovsky et al., 1981.
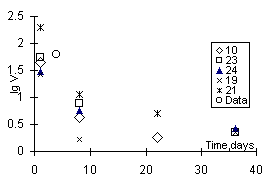
Fig.2. Leaching rate of iodine from ceramic samples based on zeolite NaX; in legend indicated numbers of runs. Data - Shidlovsky et al., 1981
The ceramics quality was evaluated from the element leaching rates in distilled water at 90oC (test MCC-1). The run duration for each sample was 1, 7, 14, and 28 days. Experimental solutions were acidified with hydrochloric acid and analyzed by ICP and atomic absorption.
Conclusion. Relatively high stability of iodine-containing zeolite ceramics was concluded from the data on leaching. The iodine leaching rate was 0.9 g/m2day after runs under the MCC-1 test conditions during 36 days. It was 4-5 times less than the rate of iodine leaching from iodosodalite obtained by hot pressing of Al and Si oxide powders in the presence of alakline metal iodide or iodate solutions [4]. It correlates with the rates of alkaline and alkaline-earth element leaching from borosilicate glasses [5]. The observed decrease of the leaching rates and concentrations suggests that the leached iodine concentration will not exceed 0.5 in
52
time, what tolerates the long-time storage of radioactive iodine in ceramics form.
Using two different zeolites (NaX and Cu-zeolite) as initial materials, one can obtain ceramics of different phase composition. In the former case, its composition roughly corresponds to the mixture of feldspar and I-containing sodalite; in the latter case - quartz, corundum, and CuI. The density of such a ceramics is somewhat higher than that of NaX-zeolite ceramics. The ceramics containing CuI proved to be twise as stable as NaX-zeolite ceramics as for iodine leaching (Figs.1 and 2, respectively).
Thus, we developed two methods of iodine fixation. The first (with initial NaX zeolite) offers the following advantages: - the obtained ceramics must be stable when burried in alkaline rocks, where the paragenesis feldspar + sodalite is abundant; - the initial zeolite is low in cost and accessible (it is industrially produced and allows secondary utilization).
The second method (with initial Cu-zeolite) offers the following advantages: - iodine is firmly fixed in CuI form, which is more resistant to leaching, compared with sodalite; - the ceramics has higher density and strength, compared with the former method.
The proposed method of conservation of radioactive iodine in the ceramics form has some advantages: the obtained material possesses high strength and significantly reduces the volume of radioactive waste. It is of principle importance that the ceramics is prepared from the industrial zeolites capable of absorbing radioactive iodine directly from the gaseous streams of nuclear plants. According to the principle of phase and chemical relations in the system matrix-rock [6], this ceramics (especially, that based on the assemblage feldspar + sodalite) is compatible with the rocks of the earth's crust (syenites) and thus excells the iodine-fixing matrixes proposed before: Ag-containing zeolite, based on epoxy glue compositions [7] etc.
References:
Suvorova V.A., Pertsov N.B., Kotelnikov A.R. Sr-containing float slime ceramics - the final product of biomineral interaction.
The aim of the work has been to synthesize a matrix material for binding strontium contained in a wet float slime being a product of biocolloidal technology of radionuclides extraction from polluted: contaminated waters. Based on a study of strontium leaching, the possibility has been estimated for using solid model compositions produced by ceramization of Sr salts-kaolinites mixtures for a long-term immobilization of 87Sr under controlled storage.
Liquid radioactive waste (RAW) left after a fuel cycle or formed in emergency are very dangerous when stored. By joint efforts Russian and Ukrainian scientists developed a method for removing radionuclides from polluted waters accumulated under the failed reactor of the fourth block of the Chernobyl AES.
The method is based on treating the water by specially selected metalphilous micro-organisms with later separation of the disperse phase (radionuclide concentrate) by floatation. A decrease of the potential danger of RAWs and of their volume is reached by compacting, i.e. solidification. We have attempted to produce a solid alumosilicate matrix from Sr-containing float slimes, i.e. products of micro-organisms floatation, through their ceramization. To this end we used a sample of wet float slime containing about 10 wt% Sr kindly given to us by the colleagues from the Institute of biocolloidal chemistry of the Ukrainian NAS where it was produced by 1-2 intermixing of active slime (micro-organisms) with a water solution of strontium chloride (200 mg/l). Strontium was bound living cells united to flocules measuring 50-100 μm.
The separation of the Sr-loaded biomass was carried out using coagulants. 50 ml of the starting float slime was dried at 110oC until a solid residue was obtained, after that the residue was burnt at 700oC. The dry residue weight was 226 mg. A microprobe analysis of the powder showed that along with oxides of silicon (4.68), aluminium (7.43), calcium (2.39), high concentration of SrO (48.12%), it contains a large amount of Cl (21.03%).
The latter implied that strontium is retained as insoluble chloride, therefore it cannot be introduced into the alumosilicate matrix which is recognized as the most stable in keeping alkali-earth elements. For ceramization strontium ought to be in the form of compounds which easily decompose at high temperatures, i.e. carbonates or nitrates. The slime treatment with nitric acid yielded a chlorine-free product, but its amount was small and insufficient for conducting further runs. We, therefore, performed model experiments on introduction of strontium into kaolinite.
The introduction of strontium was performed by way of ceramization of calcinate from SrNO3 or SrCO3 and natural kaolinites with the addition of 1 wt% water in sealed platinum capsules at temperatures 700 and 1100oC and pressure 1000 atm. The initial charge was produced by a prolong
53
crushing and then drying at 110oC of kaolinite-salt mixture. The calcination of the initial charge was performed in an open crucible at 700oC for 1-3 days. In the table are listed to conditions and the results of the runs, the formulae are derived from the microprobe analysis data.
|
Run |
Initial charge |
Ò, 0Ñ |
Ð, atm |
days |
Products |
d, g/cm3 |
P, % |
|
S-5 |
SrCO3 + kaolinite |
700 |
1000 |
3 |
(Na0.007K0,055Sr0.297)0.357(Al2.129Si2.239)4.368O24 |
2.7 |
12 |
|
S-6 |
SrCO3 + kaolinite |
1100 |
1000 |
3 |
(Na0.008K0,058Sr0.902)1.068(Al2.182Si2.114)4.297O24 |
2.3 |
23 |
|
S-7 |
SrCO3 + Kaol.Wool |
1100 |
1000 |
3 |
(Na0.038K0,009Sr0.787)0.834(Al2.060Si2.037)4.197O24 |
2.4 |
20 |
|
S-8 |
SrNO3 + Kaol.Wool |
1100 |
1000 |
3 |
(Na0.023K0,007Sr1.015)1.057(Al2.070Si1.982)4.042O24 |
2.7 |
20 |
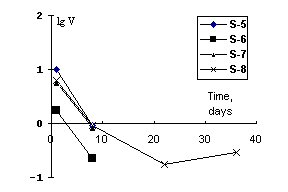
After quenching the run products were compact cylinders 30-40 mm length and 3-5 mm in dia. The X-ray patterns of the products exhibited reflexes of strontium feldspar. The ceramics quality was estimated by leaching of the elements out of the samples in distilled water at 90oC (IAAE test MCC-1). The results on strontium leaching are illustrated in the plot in the co-ordinates velocity lgV (g/m2.day) duration (day).
The results suggest that the charge from SrNO3 and kaoline wool gives the best ceramics. As little as 0.86% of strontium goes to the leaching medium in the course of the runs. The mean leaching velocity value, reached after 22 days, is 0.2 g/m2.day.
Considerable leaching velocities of Na 2.4 g/m2.day on the 40th day attest to a weak stability of this matrix material in general.
The leaching data obtained for strontium-containing ceramics on the base of the charge stoichiometrically corresponding to strontium anorthite showed that after the 40-day duration a mean leaching velocity of strontium, was 0.2 (lgV=-0.5) g/m2 that was better by a factor of 2 than from borosilicate glasses [1] with the initial strontium concentration being much higher in our samples. Despite the high porosity (~20%) dictates a rapid leaching of strontium and sodium, the sample retains a low leaching percent of Sr, that makes the use of our method promising for solidification of float slimes, that purify water basins, to the forms suitable for burial in rocks.
Reference:
Suvorova V.A., Kotelnikov A.R., Akhmedzhanova G.M. Synthesis of ceramic phosphate-containing matrices for immobilization of REE (La, Ce) radionuclides.
The aim of the work has been binding of REE (La,Ce) radionuclides, contained in nuclear fuel waste, in ceramic matrix materials by synthesizing them from phosphates, imitators of the corresponding elements, and available raw material, i.e. natural rocks.
Synthesis enables one to obtain a cheap end product that meets the principles of (1) multibarrier character of protective compositions and (2) phase and chemical compatibility in the system matrix-host rock. The produced multibarrier protective compositions consist of monazites and alumosilicates each of which is a barrier against the escape of radionuclides, through binding them chemically or mechanically, the third barrier will be lost rocks with which the synthesized ceramics is in phase and chemical compatibility (equilibrium).
The silicate components and phosphates were crushed in an agate mortar for 1.5 h until they became homogeneous in composition. 1.5 g pellets 5-6 mm in height and 8 mm in diameter were produced from the mixtures by cold compacting under a pressure of about 100 kg/cm2 at room temperature. The produced pellets were sintered in platinum crucibles for 3 days in an electric heater 'KO-14' at 1180oC.
The produced ceramic specimens had the composition corresponding to natural granite and tuff with an admixture of REE phosphates.
The leaching data obtained phosphate-silicate ceramic matrices demonstrated their high stability. After a 50-day duration under the MCC-1 test for the specimens on the base of granite the leaching rate of Ce was 2.78∙10-3 g/m2∙day, and that of La 8.24∙10-3g/m2∙day, which is comparable with elements leaching rates from synrock.
Lunin S.E. and Zhdanov N.N. Influence of subterranean explosions on migration of elements and properties of aqueous medium.
The analysis of the nuclear explosion effect on the state of the Earth crust, changes in the chemical composition of subterranean waters in places of testing were revealed along with other results. To check this assumption, logging potentiometric measurements of the chemical composition of the aqueous medium were carried out after calibrating non-
54
nuclear explosions. The studies were performed in the former Semipalatinsk testing nuclear proving ground in September-October, 1997. Observations of changing hydrochemical parameters before and after the seismic event were carried out in the hydrogeological well N 4103 during 700 h, and two measurements with a week interval from the moment of the seismic event were performed on the residue of the structural well N 1349s (depth 55 m). A PZ-64 potentiometric sound developed in IEM RAS (Patent No.), which makes it possible to detect changes in pressure (P, atm), temperature (T, C), pH, Eh, and concentrations of Na (pNa), Cl (pCl), and S (pS), was used as the measuring technique.
The measurements performed confirm the assumption about the influence of explosions on the chemical composition of water at a distance to 1-5 km from the epicenter. Jumps of all parameters measured and pressure (to 20 atm) are observed at the moment of explosion.
According to preliminary data, short-time and smoother long-time changes in the parameters measured can be distinguished in the whole time range. The pronounced changes of the first kind were observed a day and ten days after the explosion (Table 1). In the first case, the pH value decreases sharply, and the concentrations of Cl and Na increase. Ten days after the explosion, the pH values increase along with those of Cl and Na.
Long-time changes are expressed as a smooth increase in the pH value and content of Cl during 5 days, while the content of Na has a smooth tendency to decrease. Then these processes change their character to the mirror one: the concentration of Na increases smoothly, and those of Cl and pH decrease (Fig. 1).
Fig.1. The changes in water composition in drill-hole N4103 after the explosion
55
Table 1.
|
Time, h |
Depth, m |
T, oC |
pH |
|
|
|
PS |
Eh |
|
-428.65 |
-40 |
7.7 |
8.33 |
0.00 |
0.00 |
0.00 |
-252 |
-164.24 |
|
-0.01 |
-40 |
7.69 |
8.4 |
0.07 |
0.17 |
-0.33 |
-359 |
-174.30 |
|
29.34 |
-40 |
7.7 |
8 |
-0.33 |
0.61 |
-0.45 |
-338 |
-166.68 |
|
98.51 |
-40 |
7.72 |
8.34 |
0.01 |
-0.06 |
0.21 |
-334 |
-154.08 |
|
150.98 |
-40 |
7.68 |
8.33 |
0.00 |
-0.11 |
0.21 |
59 |
-137.66 |
|
174.56 |
-40 |
7.72 |
8.52 |
0.19 |
-0.25 |
0.43 |
-91 |
-144.43 |
|
193.28 |
-40 |
7.73 |
8.51 |
0.18 |
-0.40 |
0.50 |
-119 |
-131.57 |
|
225.56 |
-40 |
7.74 |
8.54 |
0.21 |
-0.30 |
0.30 |
44 |
-184.54 |
|
247.06 |
-40 |
7.72 |
8.37 |
0.04 |
0.34 |
0.47 |
-202 |
-169.45 |
|
317.84 |
-40 |
7.72 |
8.29 |
-0.04 |
0.14 |
-0.09 |
-292 |
-229.40 |
56