=FeOH2+
=FeO-
log 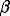
=FeOHSr2+
=FeOSrOH
log 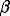
specific
capacitance
F m-2
25oC
7.39
-9.59
1.80
-17.86
2.49
50oC
7.29
-8.92
2.84
-15.53
1.62
75oC
7.37
-8.23
3.00
-14.15
1.44
+Karasyova O.N., Ivanova L.I., Lakshtanov L.Z. Acid/base reactions and Sr(II) complexation at the surface of hematite.
Understanding of adsorption of metals by common minerals is vital for predicting their transport, retardation and reactivity. Hematite is a mineral with the well-described stable surface. Its surface structure is close to that of iron hydroxides forming by weathering of base and ultra-base rocks and often controlling contaminant sorption in the subsurface environment.
90Sr forming in nuclear reactions is one of the most dangerous radioactive substances. Alongside with long half-life (28 years), it has a property to strongly be kept in the living organisms, mainly in the bones, and to slowly be removed, being a source of permanent radiation of a marrow.
Acid/base reactions and Sr(II) complexation at the hematite-solution interface have been investigated at 25, 50, and 75oC in NaCl solutions at a constant ionic strength of 0.1m. Equilibrium measurements were performed as potentiometric titrations using a glass pH-electrode. The experimental data were evaluated on the basis of the constant capacitance model. Thermodynamic model of the system hematite (=FeOH) - Sr2+ - H+ is adduced in Table 1.
Model calculations of conditions approaching natural water levels indicated that strontium adsorption and hence retardation in the soil and groundwater environment is unlikely at ambient temperature and pHs, but may be very important in disposal of radioactive waste under high pH conditions or at enhanced T (Fig.1 a, b)
Table 1. Stability constants of surface complexes
|
=FeOH2+ =FeO- log |
=FeOHSr2+ =FeOSrOH log |
specific capacitance F m-2 |
|
|
25oC |
7.39 -9.59 |
1.80 -17.86 |
2.49 |
|
50oC |
7.29 -8.92 |
2.84 -15.53 |
1.62 |
|
75oC |
7.37 -8.23 |
3.00 -14.15 |
1.44 |
Fig.1. Predominance area diagrams for Sr(II), calculated for the system hematite (=
FeOH) - Sr2+ - H+. 3 - Sr2+,
7 - =
FeOHSr2+, 10 - =FeOSrOH.
+Pivovarov S.A., Lakshtanov L.Z. Sorption of cadmium on hematite in a wide range of sorbate/sorbent ratios
Sorption of trace metal ions to metal oxides has proven to be an important process, controlling the transport and soluble concentrations in many natural aquatic systems. Cd sorption at the hematite-water interface has been studied as a function of pH in a wide range of Cd2+ concentrations. The experimental method used is a combination of potentiometric titrations with metal adsorption data. Experimental data were evaluated on the basis of the model representing a synthesis of the surface complexation (constant capacitance double layer) model and the surface precipitation one. This model satisfactorily describes Cd sorption behaviour in the whole range of solute concentration including the transition region between adsorption and bulk solution precipitation of Cd hydroxide. This region is
50
associated with formation of a surface co-precipitate where Cd is not only adsorbed onto surface sites, but forms a solid solution by structural incorporation into the underlying hematite.
Cadmium sorption by hematite results in the formation of two types of the surface complexes: --OHCdOH2+ and --OHCdOH. Intrinsic constants of the formation of these complexes are -3.28 and -5.66, correspondingly.
The experimental data can also be described by the non-electrostatic model:
logK = log[/(1-)] - log[Cd2+] - 2log[OH-]
= Cd(adsorbed)/Site density
logK = 18.54 - 2.72[11/(1+10)].
Site density of hematite was determined at 3.8 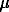 moles/m2.
moles/m2.
+Pivovarov S.A. Surface structure and site density of the oxide-solution interface.
The value of maximum adsorption is an indispensable parameter for the calculation of adsorption equilibria on the solid-solution interface. The site densities of the surface of different oxides and hydroxides measured by different authors are located in the range 1-22 nm-2. To systematize and explain these data, a new concept of 'adsorption site' has been determined. It includes one proton and one hydroxyl-ion exchangeable groups; the site densities for proton and hydroxyl-ion are equal, and maximum adsorption value corresponds to a half of number of the water molecules physically adsorbed in the first monolayer. In order to determine the site density, it is enough to know the stoichiometry of the adsorption site, the density of the mineral, as well as the lattice spacing. In the general case, the following equation is true:
Site density = d/(2nV), where d is the lattice spacing (or average lattice spacing), V is the volume per cation, n is a number of cations per site.
The values of site density for various oxides and hydroxides (n = 2) are adduced in Table 1.
Table 1. Calculated site densities of oxides and hydroxides
|
Phase |
Cation volume, Å 3 |
Face |
Lattice spacing, Å |
Site density, nm-2 |
|
Hematite Fe2O3 |
25.07 |
(001) |
2.29 |
2.28 |
|
(101) |
2.08 |
2.07 | ||
|
Goethite FeOOH |
35.15 |
(010) |
2.50 |
1.78 |
|
(120) |
2.55 |
1.81 | ||
|
(110) |
2.10 |
1.50 | ||
|
(100) |
2.32 |
1.65 | ||
|
Lepidocrocite FeOOH |
37.04 |
(010) |
3.13 |
2.11 |
|
Corundum Al2O3 |
21.13 |
(001) |
2.16 |
2.56 |
|
(110) |
2.38 |
2.81 | ||
|
(221) |
2.34 |
2.77 | ||
|
(223) |
2.08 |
2.46 | ||
|
Diaspore AlOOH |
29.33 |
(010) |
2.35 |
2.00 |
|
(100) |
2.20 |
1.88 | ||
|
(110) |
1.99 |
1.70 | ||
|
Boehmite AlOOH |
31.78 |
(010) |
2.95 |
2.32 |
|
Gibbsite Al(OH)3 |
52.72 |
(001) |
4.85 |
2.30 |
|
Rutile TiO2 |
30.94 |
(100) |
2.29 |
1.85 |
|
(110) |
3.24 |
2.62 |
51