I. Magmatic systems and petrology of the silicate melts
(Leader Ac. V.A.Zharikov)
Bezmen N.I., Kalinichev A.G., Zavelsky V.O., Zharikov V.A. Melting relations in the system NaAlSi3O8-H2O-H2 (Ptotal=2kbar)
The vapor-saturated solidus of the NaAlSi3O8-H2O-H2 system at 2 kbar over the range of gas phase composition from pure water to X(H2O)v=0.1 was studied in the internally heated gas-media pressure vessel (Fig.). In the experiments, various H2O/H2 compositions were controlled directly, rather than using solid media buffers. The results show that the melting temperatures decrease in the X(H2)v range from ~ 0 to 0.3 compared to H2O-saturated albite under relevant water fugacity. At X(H2)v=0.1 the solidus curve has a pronounced minimum with the temperature depression of 30oC. The further addition of H2 to the gaseous mixture leads to the increase of melting temperatures. In the region of WI-buffer albite melts at temperatures about 50oC higher than hydrous albite under the pure water fugacity equal to the partial one in the H2O-H2 mixture. The melting point of 1078oC in pure hydrogen has been calculated by an extrapolation of H2O-H2 data. The albite melting behavior and its dependence on the fluid composition is defined by molecular water to hydroxyl groups ratio in the melt. According to 1H NMR data, the addition of H2 to the aqueous fluid yields an essential increase of OH groups concentration in the resulting glasses compared to the hydrous ones. However, with the further increase of H2 content (X(H2)v >0.3), the amount of molecular H2O increases in comparison with H2O-saturated glasses.
Persikov E.S., Bukhtiyarov P.G. Influence of lithostatic and water pressures on the viscosity and structure of model and magmatic melts in an acidic-ultrabasic series.
The temperature and pressure dependences of the viscosity of basic melts (Di50Ab50) have been experimentally studied for the first time at P=40 kbar within the temperature range 1400-1850oC. It has been found that the temperature dependence of the melt viscosity has an exponential behaviour, the preexponential factor having a constant value. The activation energy values have been obtained under different pressures.
The pressure dependence of rheologic properties of melts of said composition has an extremal behaviour with minima of viscosity and activation energy at P=30+2 kbar i.e., to pressures <30 kbar their values decrease with growing pressure, and at P>30 kbar they grow. Earlier we found the minima of viscosity and activation energy for Di75Ab25 melts (Pmin= 12+1 kbar, Persikov, Bukhtiyarov, 1997). So the theoretical prediction was confirmed experimentally (Persikov, 1991), i.e as the basicity of magmatic melts grows the minima of the pressure dependence of their viscosity shift towards lower pressure region.
Based on the theoretical analysis of the diagrams of the pressure dependence of the viscosity and activation energy of the Di-Ab melts system which in the first approximation (without volatiles) well models acidic-basic properties and structural features of magmatic melts, we have shown that the pressure range wherein minima of the rheologic properties take place is extremely large : Pmin=105 kbar dry albite melt which models acidic magmas; Pmin ≈5 kbar dry melt of the composition Di95Ab5 which models ultrabasic magmas of, for example, picrite composition, (see Fig.1).
It has also been found that ultrabasic (metasilicate) diopside melt is a limit with respect to the melt composition (basicity) when the viscosity minimum does not show up any longer and the values of the rheologic parameters of such a melt grow with the pressure (see Fig.1 and Persikov and Bukhtiyarov, 1997, 1998).
The diagram activation energy Vs composition has been obtained for melts Ab-H2O, basalt+H2O and Di-H2O (Fig.2). An analysis of this diagram confirms the earlier about the dissolution inversion mechanism of H2O in magmatic melts as a function of their basicity, i.e. about the manifestation of the amphoteric nature of H2O at its dissolution in magmas: the dissolution of H2 in acidic-basic melts leads to their depolimerization, failure of the framework structure, i.e. to a growth of their basicity; on the contrary, the dissolution of H2O in ultrabasic melts increase the viscosity and activation energy, i.e. increase the degree of their polymerization (Persikov, 1994).
8
Fig.1. Activation energy of the viscous flow of Ab-Di and Jd melts Vs the pressure: 1 - Ab100; 2 - Ab80Di20; 3 - Ab57Di43; 4 - Ab30Di70; 5 - Di100; 6 - Jd100 (open symbols stand for extrapolated values).
Fig.2. Activation energy Vs the concentration in the systems silicate melt-H2O 1 - Ab,Ab+H2O (H2O is the base with respect to melt, experiment); 2Di,Di+H2O (H2O is the acid with respect to the melt, experiment); 3Di+H2O (H2O is an acid with respect to the melt, calculated value); 4- Di+H2O (H2O is the base with respect to the Di melt, calculation) ; 5-Basalt+H2O (H2O is the base with respect to the melt, experiment).
References:
#Khodorevskaya L.I., Zharikov V.A. Experimental study of partial amphibolite melting at various fluid phase composition.
Geochemical specialization of fluids at an early stage of their formation is largely governed by the fluid-melt interaction processes. Water fluids containing a small amount of dissolved salts affects the compositions of melts and co-existing mineral associations. High concentrations of salts in a fluid leading to a water activity decrease will change appreciably rock melting temperatures [1]. Experimental studies of the influence of the fluid phase on the basic rocks melting have long been performed but are primarily restricted to investigations of the rock-water systems. A study of the influence of a salt concentration of the fluid phase on the melt composition still has a long way to go.
We report the experimental data on the compositions of quenching glasses which we interpret as the compositions of melts, produced at a partial melting of amphibolite, with the initial composition of the fluid phase preset by NaCl solutions of various concentrations as the most important component of fluids, and also, CaCl2 and KCl solutions.
The runs were conducted at 900oC and pressure 5-7 kbar in gold capsules in a high gas pressure apparatus. The chemical composition of amphibolite and the technique for treating the run products are given elsewhere [2].
With the initial fluid phase preset by NaCl, quartz-normative quenched glasses of neutral-acidic composition form in the course of the runs. The glasses are characterized by a lower concentration of calcium and potassium as compared with glasses produced at melting of amphibolite with an excess of pure water. As the concentration of NaCl grows in the initial fluid, potassium and calcium go from the melt to the fluid phase, the trend of the melt compositions shifts towards the Na-rich region. With the initial fluid phase preset by CaCl2 of 30-60wt% concentrations the compositions of the quenched glasses are characterized by a complete absence of alkalies, the normative composition of the glass is 90% quartz and anorthite. No dark minerals are, practically, found in the composition of restite phase (at 60% CaCl2).
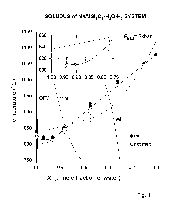
In the figure the temperature trends of the melts formed upon the amphibolite melting under the water-saturated conditions at 5-7 kbar are shown in a thin solid line. The temperature trends of the melts obtained upon melting of olivine tholeiite from Kilauea and tholeiite from Picture Gorge [3] are shown in a dashed line. It is seen from the diagram that the compositions of the melts obtained at partial melting of amphibolites of various compositions change identically with the decreasing pressure and, in general, coincide with the trends of the melt compositions obtained at dehydrational melting of amphibolite, studied by us earlier [2]. In the figure are given some data on the albite-orthoclase-anorthite relationship in natu-
# The work has been supported by the Russian Foundation for Basic Research(project 96-05-64871)
9
ral granitoids, the formation of which researchers relate to the processes of granitization of initial amphibolites evolving with the participation of high-temperature fluids. These data refer to the Archean Belomorsk [3]and Dnepropetrovsk tonalite-trondhjemite-granite (TTG) complexes [4], the Syrostan massif in the South Urals [5], amphibolite granitization zones in the southern part of the Aldan-Vytim shield [6]. The trends of natural complexes granitization are seen to coincide with the experimentally obtained trends of amphibolite conversion for the case where the present fluid was essentially aqueous. The compositions of the basic Archean (TTG) rocks also come to this realm even if their formation is related [7] not to granitization processes but to partial melting of basic rocks. Possibly, the considered trends of amphibolite conversion are basic at granitization and do not require a compositionally specific deep fluid. On the other hand from the data of [8] a mean composition of the Archen (TTG) rocks (7 in the figure) lies aside of the basic trends of amphibolite granitization and their formation can reasonably be attributed to other processes.
The introduction of KCl and NaCl into the initial fluid (shown in thick lines in the figure) leads to the corresponding shift of the melts compositions (An-Ab-Or co-ordinates). The evolution of the melt compositions in the cases of amphibolite conversion in consistency with the line CNaCl can possibly be traced for subduction zones when the sea water was captured by sinking rocks.
Fig.1. Diagram of normalized compositions of melts formed at partial amphibolite melting. In the coordinates albite-anorthite-orthoclase are given the fields: A-tonalites, B-granodiorites, C-quartz monzonites, D-trondhjemite, E-granites. The conventional symbols: 1-initial composition of amphibolite; 2-experimental melt compositions, 3-compositions of the TTG rocks from the Belomorsk complex, 4-from the Dnepropetrovsk complex, 5-from the Syrostan massif, 6-from the Aldan-Vytim shield, 7-mean composition of the Archean TTG series.
References:
#Simakin A.G., Salova T.P. Homogenic bubble nucleation in melts.
An apparatus was constructed for studying kinetics of bubble formation during a slow pressure decrease. For this purpose, a multicapsule gas vessel was combined with a special valve providing a linear decompression. The apparatus was tested in several runs with a homogeneous melt which was prepared by a special two-stage technique. It was established that bubble formation begins when a limiting pressure value is reached. At the initial water content 5.5 wt %, pressure should be reduced from 1.5 to 0.6 kbar, which is close to the theoretical value derived from the classic nucleation theory [1]. These calculations also imply that homogeneous nucleation must not occur within a real preiod of time (no more than a year) if the water content is less than 3 wt %. The experiments also indicated that mechanic processes resulting in tensile stress favor bubble formation. Bubble fronts were observed near the surface, near a spot of strong deformation of viscous melt (viscosity of granite melt in the experiments was 105-106 P), and at the boundary of diffusion dehydration accompanied by volume effects and stress. The estimates of viscous tension in the walls of a growing bubble [2] indicate that pressure in the wall (symmetric component of stress tensor) does not depend on the radial coordinate and drops to zero at a certain volume fraction 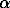 of bubbles. In two-dimension case:
of bubbles. In two-dimension case:
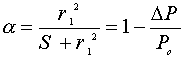
where r1 is bubble radius, S is melt area per bubble, and  P is pressure drop from the starting point at P0. Calculation of the tension values for a series of growing bubbles in terms of Maxwell (viscoelastic) rheological approximation showed that the stress field loses its radial symmetry. The propagation of the front inwards may result from the mechanic influence of growing bubbles.
P is pressure drop from the starting point at P0. Calculation of the tension values for a series of growing bubbles in terms of Maxwell (viscoelastic) rheological approximation showed that the stress field loses its radial symmetry. The propagation of the front inwards may result from the mechanic influence of growing bubbles.
This phenomenon is similar in some respect to the destruction of a porous matrix layer by layer under high fluid pressure [3]. In general, bubble formation in viscous silicate melts should be considered as a complex mechanical and chemical process.
References:
# This study was supported by the Russian Foundation for Basic Research, project no. 96-05-64262.
10
2. Simakin, A.G. and Salova, T.P., Dynamic Microregimes of Bubble Formation in Granite Melt, Fizika Zemli, 1998 (in press).
3. Aldibirov M. and Dingwell D.B. (1996) Magma Fragmentation by Rapid Decompression. // Nature, V. 380, pp. 146-149.
# Chevychelov V.Yu. Experimental study of chlorine solubility in a granitic melt: influence of melt composition alteration (granodiorite, granite, leucogranite) at T=1000oC and P=1 kbar.
The influence of melt composition alteration (granodiorite, granite, leucogranite) on solubility of chlorine in it has been experimentally studied at T=950-1000oC, P=1 kbar in the presence of initial 1m NaCl+0.1m HCl solution. The maximum content of chlorine (≈0.8-0.9 wt%) which exceed by 2-3 times the experimental data usually reported for acidic melts were obtained in granodiorite composition. In granite and leucogranite melts the concentration of chlorine decreases to ≈0.6 and ≈0.5 wt% respectively but it is still fairly high (Chevychelov, Chevychelova, 1997). The composition (in wt%) of granitoid glasses after the runs is given in the table.
|
SiO2 |
Al2O3 |
CaO |
Na2O |
K2O |
Cl |
NK/A* |
|
|
Granodiorite |
67.9 |
17.2 |
4.0 |
6.6 |
3.3 |
0.85 |
0.84 |
|
granite |
73.2 |
14.8 |
1.4 |
6.7 |
3.2 |
0.57 |
0.98 |
|
leucogranite |
75.9 |
13.4 |
1.1 |
6.3 |
2.7 |
0.47 |
0.99 |
Note: Mean of 2-4 runs, 5-8 microprobe analyses in each run. Energy-dispersion spectrometer Link. * NK/A molar ratio (Na2O+K2O) /Al2O3.
The obtained increased level of the chlorine solubility is related to both the effect of fairly high temperature and low pressure and the effect of the chemical composition of the melt, in particular, to an increased calcium concentration (Chevychelov, 1997). It can be assumed that mixed oxygen-halogenide anion groups form around Ca2+ cations in a silicate-salt melt. A decrease of the chlorine solubility in the studied granodiorite-granite-leucogranite series is principally affected by the following alterations of the composition: a) decrease of the CaO concentration 4.0-1.1 wt%; b) increase of the agpaitic coefficient (Na2O+K2O) /Al2O3 0.84-0.99; c) increase of the SiO2 concentration - 67.9-75.9 wt%.
The significance of correlations between chlorine concentration in a melt and concentrations of major elements and, also, their molar ratios has been estimated using the published data on chlorine solubility in basaltic, andesitic and phonolitic melts. It has been shown that there are correlations between concentrations of Cl and CaO, and, also, Cl and SiO2 (excluding alkaline phonolitic composition) with a confidential probability α=0.99; in the former case the correlation is positive, in the latter it is negative. Significant positive correlations have been established between Cl concentrations and such molar ratios as CaO/SiO2, CaO/Al2O3, CaO/Na2O+K2O, (Na2O + K2O + CaO)/SiO2 and the sum Na2O + K2O + CaO (+ FeO + MgO) (mol%). Negative correlation is observed between Cl and (Na2O+K2O)/Al2O3 ratio in the investigated range of compositions. This negative correlation is exchanged for the positive one at (Na2O+K2O)/Al2O3 >1.
The new data can be used for modeling the formation conditions of some ore deposits since chlorine is one of the most important complex forming agents capable of actively extracting a largest number of metals into a fluid. The complete results are reported elsewhere (Chevychelov, to be published).
References:
##Suk N.I. Silicate-carbonate differentiation in alkaline melts (experimental data).
The silicate-carbonate differentiation of melts was experimentally studied in agpaitic and calciferous systems (albite-carbonate [Na2CO3 or Na2CO3+CaCO3] and albite-diopside-carbonate) at 1100 C and 2 kbar. The experiments were performed with a high-gas-pressure apparatus; the starting materials were sealed in platinum capsules, kept for six hours at experimental parameters, and then quenched.
A wide immiscibility field was found in the studied system, where the starting melts are separated into silicate and carbonate liquids (Fig. 1). The silicate phase yields a homogeneous glass matrix in which the salt phase is present as small drops and larger clusters with sharp phase boundaries. The established heterogeneity of carbonate phases results from the immiscibility of alkaline and calciferous carbonate melts (Fig. 2). This immiscibility shows up in different ways in alkaline and calciferous systems. In the former, carbonate liquid differentiates into alkaline (sodium-rich, up to pure Na2CO3) and alkali-calciferous (calcium-rich) fractions; while in the latter, the differentiation products are alkali-calciferous fraction and almost pure calcium carbonate.
# This study was supported by the RFBR, Project N96-05-64709, and by RFBR-dfg, project N 96-05-00020G
## This study was supported by the Russian Foundation for Basic Research, project no. 97-05-64158.
11
Fig. 1. Experimental results on differentiation of melts into (1) silicate and (2) carbonate phases. Tielines connect the compositions of coexisting phases. Dashed line shows the immiscibility field at P=5 kbar and T=1250oC (Kjarsgaard and Hamilton , 1988). Compositions of rocks of natural carbonatite complexes: (3) carbonatites and nephelinites of Volcano Oldonio Lengai (Tanzania) (Dawson, 1989; Dawson et al., 1987); alkaline rocks: (4) phonolites, nephelinites, urtites, ijolites; (5) melilitites and kimberlites; (6) carbonatites of known carbonatite complexes (Kjarsgaard and Hamilton , 1988).
Fig. 2. Calc-alkaline separation of carbonate phases in the experiment (arrows): (a) alkaline and (b) calciferous systems.
The distribution of rare-earth elements (La and Ce) between the melt phases was experimentally studied. In alkaline systems, REE were found to concentrate in Ca-rich carbonatite melts, while Na-rich phases are almost free of lanthanum and cerium. In alkaline silicate-carbonate systems, REE conversely concentrate in the silicate melts rich in calcium. These results contribute to the understanding of the relation between rare-earth carbonatite deposits and alkaline agpaitic magmatism.
In magmatic evolution, calc-alkaline carbonate magmas conserve their original composition only in a volcanic setting. For example, carbonate magmas of Volcano Oldonio Lengai (Tanzania) have compositions consistent with experimental data (Fig. 1). The alkali-calciferous immiscibility in carbonate melts revealed by the experiments evidently played a certain part in the formation of carbonatite intrusions of various types. The direct separation of calciferous carbonatites from non-agpaitic silicate melts (lower part of diagram, Fig. 1) takes place in kimberlite magmatism.
The results of this study are in good agreement with data obtained at T=1250oC and P=5 kbar (Kjarsgaard and Hamilton, 1988) (Fig. 1) and properly describe the separation of carbonatite melts from agpaitic and calciferous magmas.
References:
#Salova T.P., Zavel'sky1 V.O., Epelbaum M.B. Form of water dissolved in quartz glass (PMR-investigations).
Water bearing quartz glasses with the H2O content up to 7 wt.%, obtained at T=1300oC and P=4 Kbar with the 4 hours duration are studied by PMR-method. It seems evident, that different forms of water dissolved in glass, have different sets of thermically activated mechanical degrees of freedom (rotational, vibrational, etc.). So, one can expect that a temperature behaviour of these forms of signals formed by protons in PMR-spectra will give a new interesting information on state of water in quartz glass.
On the basis of study of concentration dependence and temperature behaviour of proton signals and experiment on impulse narrowing of the PMR-line it is determined, that the basic mass of water being in quartz glass is dissociated and exists (in the studied range of water concentrations) practically only in the form of OH-groups, coordinated with silica. In quartz glasses, in contrast to alumosilicate in the limits of method sensitivity, no isolated molecules of water are discovered, which predominate in alumosilicates (up to 7 % of the dissolved water) and are represented in PMR-spectra by a distinctly distinguished typical molecular water signal - Pake`s doublet. In IR-spectra of the studied samples a band in the region of 1600 cm-1 is discovered. It correponds to the deformational vibrations of H2O molecules. However, the concentration of the molecular water by IR-spectra does not exceed (by our estimates) 3-5 wt.% of the total volume of water dissolved in the sample. The signal from such quantity of the molecular H2O on the background of the basic proton lines of
# This work was supported by the RFBR grant, project N 98-05-64148.
12
the sample in the spectrum does not manifest itself, even at a durable accumulation.
It was also determined that at T, P and exposition given in the experiment a distribution of OH-groups in the volume of the sample is inhomogeneous and in the first approximation it is nothing but embeddings of zones of enhanced density of hydroxiles into a more rather homogeneous distribution of OH-groups all over the whole volume of the sample. This inhomogeneity is of a technological origin: the samples are obtained from the SiO2 powder and water, localized in intergrain space. The time of experiment and a very low coefficient of diffusion do not allow to get a homogeneous distribution of the sample. In PMR-spectra this inhomogeneity manifests itself as a two-component proton signal: it consists of two Gaussean-Lorenz lines of a different width. The signal of OH-groups from zones of enhanced density is approximately three times wider than the line from homogeneously distributed hydroxiles.
Thus, it is shown that in quartz glass water dissolution occurs practically completely due to chemical interactions. The obtained result is important, particularly, to understand the processes of water inteaction with magmatic melts. A comparison of PMR-spectra of water bearing glasses of granite, albite and quartz compositions shows that in acidic magmatic melts water interacts with the feldspar component.
Reference:
#Litvin Yu.A., Chudinovskikh L.T. Mantle reactions and carbonate-carbon synthesis of diamond.
Melting behaviour of the system Mg2SiO4-K2CO3-Na2CO3-K2SiO3-C at high pressures is applicable to modelling hot spot interaction of chemically active plume components (K2CO3, Na2CO3, K2SiO3, C) with the matter of surrounding mantle (Fo, En) as well as to experimental imitation of diamond crystallisation at silicate-carbonate or carbonate melts of mantle origin.
Mantle hotspots are associated with mantle plumes. The plumes together with the subducting oceanic plates are the main forms of the mantle convection. Some geochemical and petrochemical data are in agreement with the idea of chemical activation of the plumes and formation of high-temperature alkaline-fluid melts enriched with alkaline, fluid, incompatible components and rare elements. The melts are able to interact with the mantle rocks forming alkaline primary magmas for the intraplate alkaline rock series including diamond-bearing kimberlites.
Olivine and orthopyroxene are the most abundant mantle minerals, and forsterite and enstatite were chosen for modelling mantle composition in the experimental systems. The simplest plume components seems to be the carbonates K2CO3 and Na2CO3 which are in the focus of present work as well as the alumosilicates NaAlSiO4 (nepheline) and NaAlSi2O6 (jadeite). The alumosilicates are chemically active in interaction with forsterite and enstatite forming a plausible mantle mineral Na2Mg2Si2O7 (NMS-phase) and pyrope Mg3Al2Si3O12 [1,2].
In the course of experimental studies of the system forsterite - K2CO3 at 3.7 GPa, next subsolidus reactions were established: (1) Mg2SiO4 + K2CO3 = MgCO3 + K2SiO3 + MgO and (2) Mg2SiO4+2K2CO3 = K2Mg(CO3)2 + K2SiO3 + MgO. The newly formed carbonates MgCO3 and K2Mg(CO3)2 melt congruently at high pressures. The subsolidus assemblies are: (i) Fo + MgCO3 + K2SiO3 + MgO, (ii) MgCO3 + K2Mg(CO3)2 + K2SiO3 + MgO, (iii) K2Mg(CO3)2 + K2CO3 + K2SiO3 + MgO. Periclase MgO is only liquidus phase. For the Fo - K2CO3 pseudobinary join, three invariant eutectic points associated with the subsolidus assemblies were revealed. Formation of carbonate phases in the course of the interaction of active plumes with the mantle suggests a possibility of intensive processes of mantle carbonation. The experimental data will allow understanding of the role of primary carbonate and silicate melts in the formation of the intraplate alkaline rock series. Preliminary experimental data on an interaction of forsterite with Na2CO3 are consistent with formation of Na2Mg(CO3)2 at high pressures.
First experimental results [3 - 5] demonstrated that alkaline-carbonate melts are good carbon solvents at high pressures and temperatures. The carbonate melts - carbon solutions are very effective for crystallisation of diamonds. Our new experiments at 8 - 10 GPa, 1600 - 1800oC on the Na2Mg(CO3)2 - K2Mg(CO3)2 - C (graphite) system led to spontaneous and seed stimulated crystallisation of diamond on the Na2Mg(CO3)2 - C and NaKMg(CO3)2 - C joins. Both synthetic metal- carbon and natural diamond seed crystals were used. For the conditions of spontaneous nucleation, colourless, transparent diamonds of octahedral habit up to 0.1 - 0.15 mm size were formed. For the conditions of seed stimulated growth, it was found that the growing layers on the octahedral faces of the seed crystals have octahedral orientation. But, the newly grown layers over cubic faces are formed with intimately contacted octahedral microcrystals. The peculiarities of growth of synthetic carbonate-carbon diamonds are similar to those for natural diamonds. The carbonate- carbon synthesis of diamond is of great interest for the problem of natural diamond genesis in the mantle conditions and for further developing synthetic diamond technology.
Referenses:
# This work is supported by the Russian Foundation of Basic Research (project N 96-05-64786) and Federal Program "Integration" (project N250).
13