II. Ore systems and processes
Marakushev A.A. Petrologic model of gold-bearing quartz vein formation.
Experimental studies carried out in the laboratory of thermodynamics of minerals of the IEM RAS in collaboration with Yu.B.Shapovalov have revealed specific features of ore concentration in magmatic systems invariably associated with segregation in them of slat fused phases that preferentially concentrate orogeneous metals by effectively extracting them from silicate melts [1,2,3, and others]. As contrasted from salt melts, hydrothermal solutions do not possess effective ore concentrating properties in magmatic systems, i.e., the concentration of ore metals in them is, as a rule, lower against silicate melts from which they segregate. So, in the evolution of magmatism the hydrothermal activity is more related with scattering factors rather than with the concentration of ore metals. This casts some doubts on purely hydrothermal hypothesis of ore deposit formation, particularly large ones, containing rich ores. Their formation is governed by salt-assisted preferential extraction of orogeneous metals from residual intrusive melts.
Ore-bearing intrusives wherein such extraction took place could form only in relatively closed physical-chemical systems where the crystallization-assisted differentiation was accompanied by effective concentration of salt components in residual melts with subsequent segregation of independent salt fused ore-concentrating phase. In intrusives differentiating under the attack of transmagmatic solution fluxes which favour the migration of highly soluble salt components from magmatic systems no segregation of independent salt fused phases occurred which may explain the absence of metallic specialization in the largest number of intrusives. Their evolution was completed with the formation of the system silicate melt-hydrothermal solution, that promoted ore metals scattering or, at best, formation of metasomatites with a poor disseminated mineralization (tin-bearing greisens, etc). Under conditions of relative isolation from transmagmatic fluid fluxes the evolution of magmatism leads to the formation of three-phase systems: residual silicate melt-salt melt, preferentially concentrating orogeneous metals, - hydrothermal solution. Concentrated ore formation models ought to be based on such three-phase systems.
We shall illustrate it by the example of the formation of ore-bearing quartz veins.
Quartz veins bearing native gold represent the principal type of endogenic gold-bearing deposits. The problem of their formation has always been most unclear in the science concerned with ore deposits. The generally accepted hydrothermal origin of ore-bearing quartz veins came under criticism (as far back as the beginning of our century) in the works of V.Lingren, a greatest expert of gold-bearing deposits. He wrote wittily that in order to hydrothermally form the gigantic gold-bearing quartz Mother vein in America one has to make pass through a crack a solution volume equal to the Mediterranean sea.
As far as the problem is concerned it has long been unclear what precisely unites quartz and native gold in processes of formation of ore veins. Our studies have shown that such a uniting factor is salt-assisted extraction of gold from residual melts accompanied by the effect of concentration in salt phases of silicon as well. Based on the extraction mechanism the process of formation of gold-bearing quartz veins can be represented as the reaction:
The reaction binds up the accumulation of gold and silicon (SiF4 +3AuCl) in salt fused phases of a residual chamber of granite or granodiorite intrusives, subsequent migration of these productive phases to joint zones together with a hydrothermal solution (3H2O) which causes the separation from it of gold-bearing quartz veins (SiO2 + 2Au) and escape residual highly soluble components (H2[AuCl3O]+4HF) that produce aureoles of geochemical scattering of gold and metasomatic alterations of host rocks in the vicinity of the ores.
So, the proposed petrologic model explains the principal attributes of gold-bearing quartz veins, genetically associated with residual chambers of granite or granodiorite intrusives including aureoles of an increased gold concentration in host rocks in the vicinity of the ores. The model is based on transformation of migration forms of gold due to its transition from univalent to trivalent state which leads to a partial deposition of gold in the form of native metal united with quartz as the temperature decreases. The transformation of migration forms has the most general significance that governs the geochemical behaviour and metallogeny of gold.
References:
#Gorbachev N.S. Sources and formation conditions of the Pt-Cu-Ni deposits (Norilsk region).
Partitioning of Cu, Ni, Co, Au, and platinoids has been studied in ores of major mineral types and, also, in
# This investigation was supported by RFBR N 97-05-65925
14
vertical and horizontal cuts of the Octyabr'sky Pt-Cu-Ni sulphide deposit (Talnakh ore deposit, Norilsk reg.)
Geochemical zonings of two types have been revealed in the Principal Sulphide Deposit (PSD). Type 1 characterizes partitioning of ore elements in ores of major mineral types which form the PSD. From Po to cupriferous ores (Cp, Mh, Tal) the concentrations of Cu,Au,Pt, Pd grow whereas those of Fe, Os, Ru, Rh, Ir decrease. The ores of principal mineral types are well distinguished in the concentration of Cu and the Cu/(Cu+Ni) ratio (see the figure).
Fig.1 Composition of major mineral ores: 1-pyrrhotine, 2-pyrrhotine-chalcopyrrhite; 3-cubanite; 4-cupriferrous (chalcopyrrhite, monchukite, talnachite), Po-pyrrhotine, Cp-chalcopyrrhite, Cb-cubanite, Pn-pentlandite, Mt-magnetite.
Fig.2. Elemental partitioning in the vertical cut of the deposit: 1-host rocks; 2-massive ores, 3-disseminated ores in intrusives. Concentrations: Cu, Ni%, Au, Pt, Pd, Ru, Rh, Ir, Os - 10-2 ppm.
Mineralogic and the associated geochemical zonings are governed by fractional sulphide melt crystallisation in the process of which Mss of compatible (Fe, Os, Ru, Rh, Ir) and incompatible (Cu,Au,Pt,Pd) elements separate.
15
Geochemical zoning of type 2 characterises the partitioning of ore elements in the vertical cut of the deposit and in the horizontal cut of the PSD. The vertical and horizontal zoning involves the enrichment in ore elements of the frontal part of the PSD and its upper exocontact (fig.2,3).
The sequence of the enrichment degree (k=1-2) < Cu < Pd < Rh < Pt <Ir < Ru (k=5-30) < Os (k=5-30) <Au (k>100) correlates with the affinity of these elements to sulphur and is independent of the affinity to Mss. The zoning formation is related to the transport of metals by fluids in the process of the sulphide magma degassing at an early deposit formation stage.
Fig.3. Elemental partitioning in the horizontal part (PSD). 1-intrusive, 2-PSD, A-A-sampling profile.
#Gorbachev N.S. Investigation of the effects of pressure and fluid composition on the partitioning of macro- and microelements between the fluid and the magmatic melt.
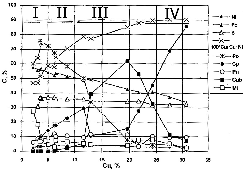
We have continued our experimental study of the effect of pressure (1-10 kbar) and fluid composition [H2O+HCl, (0.1-2N), H2O+CO2] on partitioning of the tracer elements of the fluid-magmatic systems REE, K-Na, Ba-Sr, Cu-Fe-Pb-Zn and, also, chlorine between the fluid and the melts of basic, neutral, and acidic composition. The extrema of the dependence of partitioning coefficients Df/l are clearly defined and refined for REE, chlorine, and other elements (P=5-8 kbar) (fig.1). The baric partitioning coefficients D(P)= D(P1)/ D(P2) characterising the effect of pressure on Df/l vary within 6-40. It has been shown that enhancement of the fluid acidity and temperature decrease lead to a growth of Df/l of the tracer elements. Magmophilic, hydrophilic, and indifferent elements have been revealed from a relative affinity to fluid and melt. A progressive increase of Df/l of PEE with increasing of their ordinal number, radius, and volatility at constant T-P-X parameters has been found. The found correlation of Df/l of the guiding elements with respect to affinity to chlorine and oxygen makes it possible to predict Df/l of other elements. The positive dependence of the partitioning coefficients K f/l K/Sr. Ba/Sr, Cu/Zn, Cu/Pb, Cu/Fe on the pressure, and the possibility of using the experimentally obtained pressure dependence as fluid-melt geobarometers to estimate the degassing depth of magmas of neutral and basic compositions, and the formation of ore-bearing fluids giving rise to massive sulphide ores deposits. The solubility (0.3-1.2 wt%) of chlorine in basalt, andesite, and granite melts in equilibrium with a water-chloride, water-chloride-carbon dioxide fluid in the pressure range from 1 to 10 kbar as well as the partitioning coefficients DCl between them (2-8) have been defined.
a)
b)
# This investigation was supported by RFBR N 97-05-65925
16
c)
Fig.1. Partitioning of REE between the fluid and basalt melt at T=1000oC and P=1-12 kbar. a) Df/l of *the REE at P=1,3,5,8,10, and 12 kbar. b) Pressure dependence of the Df/l of REE. c) Effect of the fluid acidity on the Df/l of REE: I-1m HCl, II-0.5 m HCl.
Fig.2. Effect of temperature on the Df/l of REE: the fluid = lamproite system, P=5 kbar. I-1100oC, II=1200oC.
Fig.3. Exchange coefficients of main trace and rare earth elements under various pressures.
Fig.4. Df/l-ionic radius Ra REE correlation.
Bezmen N.I., Naldrett A.J., Asif M. Solubilities of Pt and Pd in the hydrous silicate melt.
Palladium and platinum solubilities in silicate melt of Di55An35Ab10 composition at oxygen fugacities from HM to WI buffer at 1200oC and 2 kbar of H2O-H2 fluid pressure were determined. All experiments were run in gas-media pressure vessel under control of hydrogen portion in
17
the fluid pressure (Bezmen et al.,1991). Solid oxygen buffer assemblages (HM, Re-ReO2, MnO-Mn3O4, NNO) in double capsule experiments have been used to obtain low oxygen fugacities. At more reduced conditions different H2O/H2 compositions were directly controlled by argon-hydrogen mixtures. Hydrogen fugacities were varied from 5.2.10-2 bar (HM buffer) to 1107 bar (molar fraction of hydrogen, XH2O=0.45). Reagent-grade oxides were used to prepare the silicate glass. The glass (250-300 mg) was pressed into Pt or Pd capsules 8 mm in diameter and 50 mm long which were filled with water (250 mg) and sealed. A series of runs of different duration were carried out to determine the time necessary to attain equilibrium. After the experiment the glass samples were polished (0.3-0.5 mm) before analysis (INAA) to remove Pd or Pt contamination. Some samples were split into two parts (double runs). The first half was analyzed; the second one with Pd or Pt was again used as the initial charge in the experiment under the same conditions.
A decrease in oxygen fugacity in equilibrium with the melt brings about a considerable decrease in Pd solubility (Fig. 1) in the concerned values of fO2. The solubility behaviour of Pt depending on oxygen fugacity is more complex. In HM buffer oxygen fugacity Pt solubility is higher than Pd solubility (Fig.1). Under subsequent decrease of the oxygen fugacity, the Pt solubility as well as the Pd solubility decrease even in MW buffer. For oxygen fugacities near that produced by MW buffer at XH2O= (?) 0.1 a spasmodic increase of Pd and Pt solubilities are observed and the curves slope changed. This effect is caused by the increase of water solubility in silicate melt in the presence of hydrogen (Bezmen et al., 1991). In constrast to the behavior of Pd, Pt solubility increases at the range of oxygen fugacity from MW to WI buffer. This trend is incompatible with the usual formulation of metal/silicate melt equilibria. If metals are dissolved in silicate as cations, solubilities should decrease with decreasing fO2, if they are present as metal, solubilities should be independent of fO2. Borisov et al. (1995) have assumed that the apparently high solubility of Pt or Ir in reduced field is due to the formation of unequilibrum tiny Pt or Ir nuggets. They have sharply criticized the conclusions of Amosse et al. (1990), who also observed an increase of Pt solubility with decreasing oxygen fugacity. In order to understand this problem, we have studied the Pt-saturated silicate glasses by EPR (electron paramagnetic resonance) spectroscopy. It is possible with confidence to contend, that in the investigated interval of oxygen fugacity the Pt is in a bound state and does not form tiny Pt nuggets in the silicate glasses. The nature of the ligands to Pt in this experiment is not clear, and in any case, the features of Pt bonds will change depending on fO2, so we can not with confidence explain the increase in Pt solubility seen by other investigators under different conditions. Since the solubilities of Pt and Pd increase in hydrous silicate melts in relation to dry conditions (Borisov et al., 1995; Amosse et al., 1990), they clearly are dissolved as hydroxylic complexes. Reduced conditions promote depolymerization of silicate melts, especially in the presence of water-hydrogen fluid. This process results in the formation of clusters (Bezmen et al., 1992), probably containing platinum, that increase its solubility.
References:
#Konnikov E.G. Study of ore-forming factors of basic ore-magmatic systems.
A series of experiments on melting of pyrrhotite (Po) under a pressure of 1000 atm of a water-carbonate fluid mixture (H2O: CO2 = 1 : 1) allowed us to establish (Fig. 1) a decrease in the solidus temperature of iron monosulfide by 120-130oC relative to the beginning of its melting under "dry" conditions when the fraction of fluid in the system is 15-20 wt.% relative to the weight of pyrrhotite, which is 30-40oC higher than that under the same conditions of melting of pyrrhotite with water (Konnikov, 1997). The quenching products of these experiments contain, along with pyrrhotite, a new phase magnetite, whose amount increases proportionally to the content of the water-carbonate mixture relative to the pyrrhotite in their blend. This allows one to relate the observed decrease in the melting temperature of iron monosulfide to the oxidizing effect of the fluid on the pyrrhotite melt. This conclusion is confirmed by the results of the thermodynamic studies of the system Po + H2O + CO2 by the Selector-S program (developed by G. I. Karpov and K. V. Chudnenko) performed by G. A. Pal'yanova in UIGGM SB RAS (Fig. 1).
Fig.1. Result of the experimental exploration of Po + H2O + CO2 system under Ptot=1 kbar.
# The works were supported by the Russian Foundation for Basic Research (project no. 96-05-64714).
18
Fig.2.Distribution of reduced (I), oxidized (II) and inert (III) gases at the Dovyrenskii massif cross-section.
The study of the fluid regime of the formation of Cu-Ni-PGE-mineralization in mafice-ultramafice complexes was continued. The composition of gases conserved in pores and microinclusions during crystallization of layered Dovyrenskii dunite-tractolite-gabbro massif (the Northern Nearbaikalia) was studied by chromatography and mass spectrometry. The reduced (CH4 + H2) fluid composition of gases in this pluton and their maximum concentration in the horizon of taxitic troctolites and anorthosites with the predominant noble-metal mineralization of the "low-sulfide" type were established (Fig. 2).
#Suk N.I. Experimental study of distribution of REE (La, Ce), Nb, and Ta between immiscible phases in silicate-salt systems.
Aluminosilicate melts with added ore components (Nb2O5, Ta2O5, TiO2, La2O3, CeO2) and salts (NaCl and NaPO3) were experimentally studied in sealed platinum capsules with a high-gas-pressure apparatus at 1250oC and 2 kbar.
The experiments indicated a regular differentiation of the starting melts into immiscible silicate and salt (chloride or phosphate) liquids, which were present as layers separated by a clear phase boundary.
While phosphorus is closely related to aluminosilicate differentiation in alkaline intrusions (relatively poor in silica, e.g. urtite), chlorine characterizes the migration of ore elements from these intrusions. This different behavior is the result of higher solubility of chlorides in aqueous solutions compared to phosphorus salts and weaker bonds of chlorine with aluminosilicate melts compared to phosphorus. Chlorine is referred to as a magmaphobic component by its properties, while phosphorus shows a pronounced magmaphilous behavior.
In experimental runs, this difference is manifested in the mechanism of separation of salt phases from aluminosilicate melts. Pure salt melts (NaCl), which are separated in chloride systems, concentrate ore metals selectively, whereas phosphorus melts are typically complex in composition and enriched in ore and petrogenic metals.
The chloride extraction of metals, as any other kind of salt extraction, is selective, which was established previously for the same system with added titanium and zirconium. This study concerns rare-earth elements (La, Ce), niobium, and tantalum. The experiments showed that silicate melt is enriched in cerium compared to its starting composition (Table 1), whereas the behavior of lanthanum is ambiguous and depends on the starting composition of the system. As the lightest rare-earth element, La is partially extracted by chloride melt. In diopside-bearing systems, the silicate phase is depleted of lanthanum compared to the starting compositions. The salt phase (NaCl) proved to contain inclusions rich in Ca, Mg, La, and Ce (probably chlorides of these elements). Niobium and tantalum are concentrated in the silicate melt (Table 2).
The distribution of REE (La and Ce), niobium, and tantalum between the silicate and salt melts was also studied in silicate-phosphate systems. REE, Nb, and Ta were found to enrich the phosphate melt (see figure).. If titanium is present in the system, these elements are concentrated together .
Thus, our experimental results illustrate the indifferent behavior of Nb, Ta, and rare-earth elements in the chloride extraction, which radically differs from the efficient phosphate extraction of these elements.
# This study was supported by the Russian Foundation for Basic Research, project no. 97-05-64158.
19
Table 1. Compositions of silicate glass samples in silicate-chloride systems with REE (at %).
|
N |
Si |
Al |
Mg |
Fe |
Na |
K |
Ca |
Cl |
La |
Ce |
|
Start 1 |
33.23 54.76 |
7.81 12.81 |
4.23 6.68 |
0.43 0.02 |
28.11 16.79 |
- 0.06 |
4.80 5.70 |
20.77 2.34 |
0.32 0.31 |
0.30 0.53 |
|
Start 2 |
39.00 59.31 |
11.15 16.77 |
2.03 3.13 |
0.21 0.08 |
27.65 15.10 |
- 0.09 |
2.31 2.60 |
17.07 1.75 |
0.30 0.32 |
0.29 0.84 |
|
Start 3 |
35.74 64.16 |
11.47 20.31 |
- - |
- - |
31.56 12.76 |
- 0.03 |
- 0.09 |
20.62 1.32 |
0.31 0.34 |
0.30 0.99 |
|
Start 4 |
32.11 64.82 |
10.30 20.73 |
- - |
- - |
33.38 11.52 |
- 0.02 |
- 0.07 |
23.55 1.38 |
0.34 0.58 |
0.32 0.88 |
|
Start 5 |
36.59 53.13 |
7.61 11.18 |
6.14 8.50 |
0.62 0.08 |
24.29 15.68 |
- 0.05 |
6.97 7.92 |
17.18 2.66 |
0.31 0.17 |
0.29 0.62 |
|
Start 6 |
42.14 63.93 |
10.22 14.63 |
2.23 2.81 |
0.23 0.18 |
25.84 13.79 |
- 0.07 |
2.53 2.20 |
16.15 1.69 |
0.33 0.21 |
0.32 0.49 |
Table 2. Compositions of silicate glass samples in silicate-chloride systems with Nb and Ta (at %).
|
N |
Si |
Al |
Mg |
Fe |
Na |
K |
Ca |
Cl |
Nb |
Ta |
|
Start 1 |
41.47 54.13 |
9.71 12.26 |
5.28 6.44 |
0.53 0.07 |
21.85 16.41 |
- 0.10 |
5.99 5.43 |
12.68 2.35 |
1.49 1.61 |
0.99 1.22 |
|
Start 2 |
46.72 57.91 |
13.35 15.84 |
2.44 3.05 |
0.24 0.02 |
22.44 16.36 |
- 0.06 |
2.76 2.50 |
9.76 1.67 |
1.37 1.45 |
0.91 1.13 |
|
Start 3 |
44.51 61.75 |
14.29 18.61 |
- - |
- - |
26.19 14.50 |
- 0.06 |
- 0.06 |
12.56 1.61 |
1.48 2.18 |
0.98 1.68 |
|
Start 4 |
41.26 62.51 |
13.24 18.94 |
- - |
- - |
27.70 12.04 |
- 0.05 |
- 0.07 |
15.07 1.13 |
1.64 2.93 |
1.09 2.33 |
|
Start 5 |
43.89 52.34 |
9.13 10.57 |
7.37 8.12 |
0.75 0.04 |
18.37 15.13 |
0.00 0.06 |
8.36 7.68 |
9.84 2.67 |
1.38 2.31 |
0.92 1.07 |
|
Start 6 |
38.34 51.86 |
5.13 9.05 |
10.65 9.04 |
1.08 0.05 |
17.41 14.49 |
0.00 0.06 |
12.09 8.79 |
12.79 2.21 |
1.50 2.82 |
1.00 1.61 |
Fig.1. Distribution of ore metals between immiscible phases in silicate-phosphate systems: a - La (1) and Ce (2); b - Nb (3) and Ta (4) from experimental data.
20
#Bogolepov M. V. Experimental study on the influence of the composition of model fluid on the composition of melts.
The fluid generated in the undercrust zones of the Earth plays an important role in the formation of granite melts. Under conditions of temperature and pressure decrease, the action of these fluids results in nonisochemical melting of rocks by treating them with the plutonic fluid, i.e., in granitization.
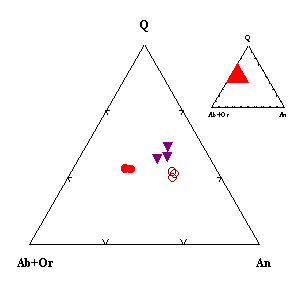
Fig.1. Influence of fluid composition on the composition of first melts in the system amphibolite - H2O HCl under fluid decompression from 6 up to 4 kbar, T=750oC. ● - H2O;▼ - 0.5 m HCl; ○ - 1m HCl.
The purpose of the work is the experimental study of the composition of forming acidic melts depending on the composition of the model plutonic fluid when HCl concentration in it changes. It is of interest for studying the possible effect of this fluid from the viewpoint of the effect of acid-base interaction. The previous works devoted to experimental studying this effect (Kuznetsov, Epelbaum, 1985) were carried out on model systems. In this case, this effect was studied in the multicomponent system.
The procedure of two-stepped isothermal pressure decrease (Zharikov et al., 1990) was used for saturation of a solution with components of the starting blend: at the first stage of the experiment, the components were leached from amphibolite; at the second stage, the melt was formed. The Baikal amphibolite was used as the starting rock. In experiments with decompression, the melt was formed on labradorite crystals. The solution : weighed sample ratio was 1 : 1. The following solutions were used: H2O; 0.5 N HCl; and 1 N HCl. All experiments were carried out on a high-pressure hydrothermal apparatus with external heating at T = 750oC and pressure drop from 6 to 4 kbar. The duration of experiments was 7 days at P = 6 kbar and 1-2 days at P = 4 kbar. Compositions of melts and crystals of newly formed plagioclases after experiments were determined on a microanalyzer.
In the experiment, both the amphibolite charge and labradorite (Hornblend : Pl ~ 1 : 1) are incongruently dissolved. The experimental data on solubility of basic and ultrabasic rocks in fluids at high parameters are presented in many works (Zharikov et al., 1990) showing the high solubility of SiO2 and alkalis in the fluid. In our experiments, we determined the composition of the dry residue of the fluid equilibrium with plagioclases with different compositions. (Bogolepov, Salova, 1995). It is established that only the albite component goes to the solution. The fluid is unloaded when the pressure decreases, due to which acidic melts are formed. No melting occurs without pressure drop.
The refined results of experiments recalculated using the CIPW system are shown in Fig.1 and show that the compositions of the melts formed are enriched in the anortite components with a decrease in the content of albite and orthoclase in the products of experiments as the acidity of the fluid increases during granitization of the amphibolite. This corresponds to the published data (Marakushev, 1973) on basicity indices of different phases of the granite system. It is noted that after experiments pH of the solutions changed from the acidic one to approximately 3-5. This change is due to leaching of Ca, Na, and K with an acidic solution from hornblende and plagioclase. It is also observed that after experiments labradorite is enriched in the anortite component, and the newly formed plagioclase has the bytownite-anortite composition. After experiments, the content of chlorine increases in the melts to 0.12 and 0.19 wt.% as the concentration of HCl in solutions increases from 0.5 N to 1 N, respectively. The greater the increase in the content of Cl, the greater the decrease in the crystallization field of plagioclase as the more basic one in the melt, and the field of K-Na feldspars is extended.
Thus, the experiments on decompression of the fluid with the complex composition show that the action of HCl shifts the point-minimum of the system toward calcium plagioclase, reflecting the acid-base interaction of the components in the melt. The crystallization field of more "acidic" feldspars is extended.
References:
# This wark was supported by the Russian Foundation for Basic Research, project no. 96-05-64710.
21