Phase equilibria (transformations) under high P-T parameters
# Litvin Yu.A., Gasparik T., Tikhomirova V.I., and Chichagov A.V. Na-Mg-silicates as plausible minerals of the Earth's mantle: melting and structural stability at 1 atm and high pressures.
key words [Na-Mg-silicates phase transformation melt]
Interest in Na-Mg-silicates as plausible mantle minerals was aroused by the discovery of high-pressure reaction between forsterite (Mg2SiO4) and jadeite (NaAlSi2O6) at pressures above 4.5 GPa (Litvin and Gasparik, 1995). Forsterite interacts with jadeite through the reaction:
4Mg2SiO4+2NaAlSi2O6=3MgSiO3+Mg3Al2Si3O12 +
+Na2Mg2Si2O7,
where the Na-Mg-silicate of Na2Mg2Si2O7 composition is formed in addition to enstatite and pyrope. Na2Mg2Si2O7 is a new compound of experimental mineralogy at high pressures, and its existence on the solidus of alkaline olivine-normative mantle as an individual phase is highly probable (Gasparik and Litvin, 1996).
Na2Mg2Si2O7 and other Na-Mg-silicates (Na2Mg2Si6O15 and Na2MgSiO4) of interest as plausible mantle minerals were experimentally studied by differential thermal analysis (DTA), high-pressure quenching, microprobe, and X-ray diffraction methods.
Crystalline powders of Na-Mg-silicates, which were obtained by recrystallization of stoichiometric
34
gels at 700oC for 1 day, were used as starting materials for the thermal analysis. All the compounds studied melt congruently at 1 atm. Melting temperatures determined were with accuracy of +5oC. Enthalpies of melting  Ho(melt) of these compounds were found by comparison of the areas of respective endothermal DTA peaks and reflexes of a standard (Na2SO4 was used as the standard). Entropies of melting
Ho(melt) of these compounds were found by comparison of the areas of respective endothermal DTA peaks and reflexes of a standard (Na2SO4 was used as the standard). Entropies of melting  So(melt) were also determined. The enthalpies of melting were estimated with in +15% accuracy. The results of DTA measurements are given in Table 1.
So(melt) were also determined. The enthalpies of melting were estimated with in +15% accuracy. The results of DTA measurements are given in Table 1.
Table 1. Data on melting of Na-Mg-silicates at 1 atm.
| |
T, oC |
T, K |
 Hmelto kJ/mol Hmelto kJ/mol
|
Smelto entr. units |
|
Na2Mg2Si2O7 |
970 |
1243 |
59.65 |
11.46 |
|
Na2Mg2Si6O15 |
1010 |
1283 |
50.80 |
9.45 |
|
Na2MgSiO4 |
1320 |
1593 |
34.17 |
5.12 |
Table 2. Melting temperatures (oC) of Na2Mg2Si2O7, Na2MgSiO4, and jadeite (Na2AlSi2O6) in dependence of pressure.
|
P, GPa |
Na2Mg2Si2O7 |
Na2MgSiO4 |
NaAlSi2O6 |
|
4 |
1400 |
- |
1650 |
|
9 |
- |
1670 |
2020 |
|
10 |
1750 |
- |
2080 |
|
13 |
- |
1790 |
2260 |
The dependences of melting points of Na2Mg2Si2O7 and Na2MgSiO4 on pressure in the pressure range up to 20 GPa were experimentally studied with a high-pressure split-sphere multianvil press. Some results are presented in Table 2 together with data on melting of jadeite (alkaline high-pressure mantle component) (Litvin and Gasparik, 1993).
These data indicate that the melting points of alkaline Na-Mg-silicates are by 250-560 degrees lower than the melting point of jadeite in the pressure range 4-16 GPa. Participation of Na-Mg-silicates in the solidus parageneses of mantle peridotites is responsible for some peculiar properties of the mantle magma formation, including generation of alkaline magmas.
Some high-pressure experiments were performed with the apparatus “anvil-with-hole” to identify high-pressure polymorphism. X-ray diffraction analysis of quenched Na2Mg2Si2O7, Na2Mg2Si6O15, and Na2MgSiO4 samples after the five-hours runs at 3.7 GPa and 1200oC and 30-min runs at 7.0 GPa and 1300oC indicated polymorthic transition of Na2Mg2Si2O7 as a result of pressure effect. X-ray microanalysis showed that the samples were single-phase and were represented by the stoichiometric compound Na2Mg2Si2O7. The other compounds disproportionate to Na2SiO3 and MgSiO3 or MgO. Therefore, these substances underwent polymorphic and chemical transitions, and the high-pressure phase Na2Mg2Si2O7 proved to preserve at metastable conditions.
Kulinich S.A., Zhukov A.N., Burdina K.P., and Semenenko K.N. ( Polymorphic modifications in the system Mg3N2-BN at high pressures.
key words [polymorphic modifications Mg3N2-BN]
The interaction of magnesium and boron nitrides was repeatedly studied. It was established that the only compound forming in the system Mg3N2-BN at atmospheric pressure is magnesium boronitride Mg3BN3 (L). When heated at pressures of more than 20 kbar, the hexagonal modification of Mg3BN3 (L) was shown to undergo polymorphic transformation into the high-pressure tetragonal phase Mg3BN3 (H).
Several papers describe at least one more compound forming in this system at high pressures. The composition, X-ray characteristics, and stability field of this phase (phases) are hotly debated. To this end, the objective of the present work was to study phase relations in the system Mg3N2-BN at high pressures, primarily in the boron nitride-rich area.
The standard high-pressure cell of toroid type was used. Boron nitride and magnesium nitride or boronitride mixtures were heated up to 1600oC at 15-60 kbar for several hours (with an intermediate grinding). The resulting powders were analyzed by X-ray diffraction. The sample densities were determined by hydrostatic weighing in CCl4.
Formation of a new phase previously unknown was fixed within the pressure range 15-40 kbar (new phase 1). Sufficient purity of this compound was only attained at 15 kbar and the charge composition close to Mg3B2N4. The substance thus obtained was almost free from Mg3BN3 admixture, but contained some quantities of unreacted boron nitride and magnesium nitride, judging from X-ray diffraction data. The attempts to obtain the samples of better purity by increasing the run time and varying the initial
35
charge composition or synthesis conditions were unsuccessful.
When the X-ray pattern of Mg3B2N4 (new phase 1) was indexed, the hexagonal syngony with the unit cell parameters a = 5.397(2)Å, c=10.585(5)Å, and v = 267.0 (3 Å were assumed. The experimental density determination gives 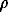 exp = 2.67(5) g/cm3 which is in agreement with the roentgenographic density of the sample (
exp = 2.67(5) g/cm3 which is in agreement with the roentgenographic density of the sample (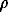 theor=81 g/cm3). The lower experimental density value is probably due to the initial boron nitride (
theor=81 g/cm3). The lower experimental density value is probably due to the initial boron nitride (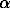 -BN) impurity in the analyzed sample. The density of this compound is significantly lower. The De-Wolf parameter M(20) is 15.3 which suggests that the X-ray indexing is correct. Obtained magnesium boronitride (Mg3B2N4 - new phase 1) appears as a brick-red substance gradually hydrolyzed on air. When heated in nitrogen atmosphere up to 600oC, it remains unchanged, but at 900oC decomposes to yield Mg3BN3 (L) and boron nitride:
-BN) impurity in the analyzed sample. The density of this compound is significantly lower. The De-Wolf parameter M(20) is 15.3 which suggests that the X-ray indexing is correct. Obtained magnesium boronitride (Mg3B2N4 - new phase 1) appears as a brick-red substance gradually hydrolyzed on air. When heated in nitrogen atmosphere up to 600oC, it remains unchanged, but at 900oC decomposes to yield Mg3BN3 (L) and boron nitride:
Mg3B2N4 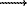 Mg3BN3 (L) +
Mg3BN3 (L) + 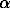 -BN
-BN
Another phase (new phase 2) of assumed composition Mg3B2N4 was established in the reaction products within the pressure interval 40 - 60 kbar. The attempts to extract this phase in the pure state were not successful. Its composition, properties, and structural features will be discussed in later publications. The X-ray diffraction data on the two new magnesium boronitrides of the assumed composition Mg3B2N4 (new phase 1 and new phase 2) compared to the previous data on known boronitrides Mg3BN3 (atmospheric and high pressures) are presented in Table 1. Both new phases and the known Mg3BN3 (H) are likely to play a major part in catalytic transition of the hexagonal graphite-like 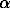 -BN into the cubic diamond-like
-BN into the cubic diamond-like 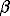 -BN that occurs in the Mg3N2-BN system at elevated tempartures and pressures.
-BN that occurs in the Mg3N2-BN system at elevated tempartures and pressures.
Table 1. Comparison of X-ray diffraction data on two new high-pressure magnesium boronitrides with previous data
|
Mg3N3B(L) |
Mg3N3B(H) |
Mg3B2N4 (new phase-1) |
Mg3B2N4 (new phase-2) |
|
d,Ao |
I/Io, % |
d,Ao |
I/Io, % |
d,Ao |
I/Io, % |
d,Ao |
I/Io, % |
|
8.04 |
23 |
|
|
|
|
|
|
| |
|
7.79 |
9 |
|
|
|
|
| |
|
|
|
5.304 |
3 |
6.732 |
1 |
| |
|
|
|
4.679 |
22 |
4.754 |
6 |
|
4.01 |
5 |
3.86 |
8 |
|
|
3.923 |
3 |
| |
|
|
|
3.511 |
43 |
|
|
| |
|
|
|
|
|
3.306 |
22 |
|
3.07 |
30 |
|
|
|
|
3.264 |
17 |
|
3.02 |
100 |
|
|
|
|
2.976 |
50 |
| |
|
|
|
|
|
2.805 |
11 |
|
2.66 |
38 |
|
|
2.700 |
18 |
|
|
| |
|
|
|
2.646 |
21 |
2.638 |
2 |
| |
|
|
|
2.616 |
28 |
|
|
| |
|
2.572 |
32 |
|
|
2.555 |
31 |
| |
|
|
|
|
|
2.513 |
11 |
|
2.434 |
38 |
2.424 |
72 |
2.409 |
14 |
2.434 |
100 |
| |
|
|
|
2.336 |
21 |
|
|
| |
|
|
|
2.305 |
12 |
|
|
|
2.215 |
7 |
2.199 |
100 |
|
|
2.220 |
14 |
| |
|
|
|
2.145 |
100 |
2.140 |
17 |
|
2.013 |
18 |
|
|
|
|
2.097 |
12 |
|
2.000 |
21 |
|
|
|
|
|
|
| |
|
1.924 |
21 |
|
|
1.930 |
12 |
| |
|
1.911 |
16 |
1.890 |
32 |
1.881 |
6 |
|
1.833 |
20 |
|
|
|
|
1.828 |
5 |
|
1.771 |
66 |
|
|
1.764 |
13 |
1.770 |
21 |
Zhukov A.N., Burdina K.P., Semenenko K.N. Novel compounds in the system BN-AlN-Mg3N2 at normal and elevated pressures.
key words [nitrides synthesis high pressure]
Urgency. Magnesium and aluminium nitrides are good catalysts for the 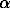 -
-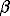 transformation in boron
transformation in boron
36
nitride, however, the phase diagrams of the systems metal nitride-boron nitride did not attract much attention with researchers until the last decade. Some data are available for the systems AlN-BN and Mg3N2-BN [1,2], and no data exist on the interaction in the system Bn-AlN-Mg3N2.
Goal. The goal of this work is an X-ray study in the system BN-AlN-Mg3N2 at normal and elevated pressure.
Technique. A mixture of the starting nitrides in preset molar ratios was heated under normal and elevated (30-75 kbar) pressures in hermetically sealed refractory steel capsules at 1200oC for 50-100 h and at 1600oC for 15-60 min, respectively. All the preparatory work with the samples was performed in a “dry” box.
AlN and Mg3N2 synthesized under laboratory conditions were used as the starting ones. The samples were subjected to X-ray (DRON-2) and microcrystalline optic examination.
The density was determined by a method of hydrostatic weighing.
Results. 37 mixtures of various compositions have been studied. It has been found that in the binary subsystem AlN-Mg3N2 under normal pressure there form five compounds having the wurtzite-type crystalline structure and belonging to the homologic series Mg3AlnNn+2, where n assumes the values from 1 to 4 at normal pressure, and from 2 to 5 at elevated one. The layering of these compounds is (n+3)Z, where Z is the number of the formula units per cell. The individual compound Mg3AlBN4 forms in the system BN-AlN-Mg3N2 at normal pressure. At pressures in excess of 50 kbar Mg3AlBN4 becomes unstable, at temperatures above 1200oC it decomposes by the reaction:
P,t
Mg3AlBN4 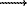 Mg3Al3N5 + Mg3B2N4 + Mg3BN3.
Mg3Al3N5 + Mg3B2N4 + Mg3BN3.
Table. Some characteristics of the compounds in the system BN-Al-Mg3N
|
Characteristic |
Mg3AlN3 |
Mg3Al2N4 |
Mg3Al3N5 |
Mg3Al4N6 |
Mg3Al5N7 |
Mg3AlBN2 |
|
a, Å |
3.368(1) |
3.287(2) |
3.257(1) |
3.239(1) |
3.231(2) |
3.439(1) |
|
c, Å |
25.05(2) |
10.75(1) |
26.36(1) |
15.60(1) |
36.22(4) |
31.43(1) |
|
Packing layering |
12 R |
5H |
12H |
7H |
16H |
|
|
V, A3 |
250.5(4) |
100.6(2) |
242.2(3) |
141.7(2) |
327/3(8) |
322.0(3) |
|
Z |
3 |
1 |
2 |
1 |
2 |
3 |
|
M20 |
29.6 |
29/4 |
16.5 |
22.1 |
6.9 |
22.4 |
|
Dexp, g/cm3 |
2.80 |
3.05 |
3.05 |
3.10 |
3.10 |
2.53 |
|
DX-ray, g/cm3 |
2.82 |
3.02 |
3.07 |
3.10 |
3.10 |
2.58 |
|
 V, % V, %
|
+1.1 |
-2.8 |
-2.7 |
-2.5 |
-1.6 |
+6.6 |
The volumetric effect of this reaction is 9.4 %. Isothermal joins of the system have been plotted at normal (1 atm) and elevated (50 kbar) pressures.
References:
- Zhukov A.N., Burdina K.P., Semenenko K.N. (1994) // Jour. Organ. Khimii., V.64, Iss.3, pp.357-360.
- Polushkin N.I., Burdina K.P.et al. (1989) // Dokl. AN SSSR, V.306, N.6, pp.1413-1420.
37
Previous Contents Next
 Ho(melt) of these compounds were found by comparison of the areas of respective endothermal DTA peaks and reflexes of a standard (Na2SO4 was used as the standard). Entropies of melting
Ho(melt) of these compounds were found by comparison of the areas of respective endothermal DTA peaks and reflexes of a standard (Na2SO4 was used as the standard). Entropies of melting  So(melt) were also determined. The enthalpies of melting were estimated with in +15% accuracy. The results of DTA measurements are given in Table 1.
So(melt) were also determined. The enthalpies of melting were estimated with in +15% accuracy. The results of DTA measurements are given in Table 1.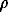 exp = 2.67(5) g/cm3 which is in agreement with the roentgenographic density of the sample (
exp = 2.67(5) g/cm3 which is in agreement with the roentgenographic density of the sample (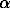 -BN) impurity in the analyzed sample. The density of this compound is significantly lower. The De-Wolf parameter M(20) is 15.3 which suggests that the X-ray indexing is correct. Obtained magnesium boronitride (Mg3B2N4 - new phase 1) appears as a brick-red substance gradually hydrolyzed on air. When heated in nitrogen atmosphere up to 600oC, it remains unchanged, but at 900oC decomposes to yield Mg3BN3 (L) and boron nitride:
-BN) impurity in the analyzed sample. The density of this compound is significantly lower. The De-Wolf parameter M(20) is 15.3 which suggests that the X-ray indexing is correct. Obtained magnesium boronitride (Mg3B2N4 - new phase 1) appears as a brick-red substance gradually hydrolyzed on air. When heated in nitrogen atmosphere up to 600oC, it remains unchanged, but at 900oC decomposes to yield Mg3BN3 (L) and boron nitride: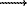 Mg3BN3 (L) +
Mg3BN3 (L) + 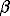 -BN that occurs in the Mg3N2-BN system at elevated tempartures and pressures.
-BN that occurs in the Mg3N2-BN system at elevated tempartures and pressures.