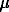 m. In the largest number of the cases the contours of these patterns are distorted which is manifested in the loss of linearity of the sides although individual portions of etch pit contours are parallel to the crystal edges.
m. In the largest number of the cases the contours of these patterns are distorted which is manifested in the loss of linearity of the sides although individual portions of etch pit contours are parallel to the crystal edges.Phase equilibria (transformations) under high P-T parameters
# Kalinin A.A., Sonin V.M., Turkin A.I. Alteration of micromorphology of synthetic diamonds under temperatures 1700-2200oC and pressure 70-75 kbar.
key words [synthetic diamond etch pit]Institute of mineralogy and petrography SB RAS
More ancient age of diamonds from kimberlites compared with their host rock indicates that they were inherited by the kimberlite magma in the process of contamination (hybridization) from earlier mantle formations. Their considerable morphologic diversity with respect to individuals found in ultrabasite xenolytes is in conformance on the whole, with the increased role played by volatiles at the formation of kimberlites, since the morphology of diamond is a good indicator of the physicochemical conditions.
Because of the paucity of experimental data on dissolution (stability) of diamond under the mantle PT-parameters corresponding to the field of its thermodynamic stability it is hard to tell presently whether the postgrowth alteration of the diamond crystal morphology is solely a result of their evolution in the kimberlite medium or it may be, in part, inherited.
So, the aim of this work was to study the morphologic singularities of synthetic crystals of diamond under elevated pressures (70-75 kbar) and temperatures (1700-2200oC) in the field of its stability.
The runs were performed in a multipunch high pressure apparatus of the 'split sphere' type with the working chamber of cubic configuration produced from refractory oxides and furnished with a cylindric graphite heater. The duration of the runs was 3-4 h. The initial material was synthetic diamonds which were placed into an MgO cylinder. The initial crystals had octahedral or cubooctahedral habit plane and measured to 0.3 mm. No surface structures were present on the faces.
After the runs the crystal remained edged and, in the basic mass, plane-faceted. Etching was manifested insignificantly. On {100} faces there appeared corrosional frosting consisting of geometrically irregular and crystallographically nonoriented etch pits. The distribution of the frosting is irregular spotty. No geometrically regular etch pattern's associated with the face symmetry were found. As contrasted from a {100} face, {111} faces demonstrated flat-bottomed negative trigons with the linear sizes 1-9 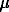 m. In the largest number of the cases the contours of these patterns are distorted which is manifested in the loss of linearity of the sides although individual portions of etch pit contours are parallel to the crystal edges.
m. In the largest number of the cases the contours of these patterns are distorted which is manifested in the loss of linearity of the sides although individual portions of etch pit contours are parallel to the crystal edges.
No correlation exists between the degree of diamond etching and the temperature. This is likely to be associated with the fact that etching occurred due to trapping of oxygen on assembling the reaction volume.
# The work has been supported by the Russian Foundation for Basic Research (project 97-05-65287)
58
The graphite heater provided the dynamic equilibrium at the CCO buffer level, therefore the etch patterns appeared apparently at the initial stage of the runs. The closeness of the fluid and redox regimes in the runs to the CO buffer level introduced irregularity into the configurations of etch pattern's on {111} faces. This phenomenon is khown under the metastable for diamond conditions (Sonin et al, 1994). It is believed that diamond etching in silicate melts occurs due not to dissolution, but through oxidation by volatiles dissolved in the melt (Rudenko et al, 1979). Solid carbon is unstable given high concentrations of both oxygen and hydrogen (Kadik, Lukanin, 1986). The experiments performed show that diamond can be stable under the pressures and temperatures corresponding to the mantle ones.
References:
#
Pal'yanov Yu.N., Gusev V.A. , Borzdov Yu.M. , Khokhoryakov A.F. , Sokol A.G. , Kupriyanov I.N. Formation conditions of the A- and C-centers in diamond in the processes of growth.key words [diamond growth defects and impurities nitrogen].
The largest number of synthetic diamonds are confined exclusively to the Ib type as they contain nitrogen in the form of individual substitutional atoms (C-center). Natural diamonds (95-98%) are confined to the Ia type and contain nitrogen preferentially in the form of A,B1, B2-centers. According to the currently dominant 'anneaing' model, the initial form of incorporation of nitrogen impurity into the diamond structure is the C-centers from which under thermal actions there form A,B1,B2 faults [1,2]. The model is based on the experimental data on annealing natural and synthetic diamonds within 1600-2600oC which are extrapolated to the region of lower temperatures down to 900oC and are at present extensively used as a geological chronometer of the processes of natural diamond formation.
The experiments of the seed growth of diamond in the basic system Ni-Fe-C were conducted in a high pressure apparatus BARS using the technique of [3]. In the first experimental series (fig.1) it was found that as the growth temperature is increased in the interval of 1400-1750oC the regular change of the type of diamond occurs in the sequence 1b  1aA (mass growth rate 3-3.5 mg/h). These data alone do not enable one to answer the question of what governs the preferential formation of A-centers - is it growth conditions or high pressure annealing?
1aA (mass growth rate 3-3.5 mg/h). These data alone do not enable one to answer the question of what governs the preferential formation of A-centers - is it growth conditions or high pressure annealing?
The results of the investigation of single crystals grown in the interval of mass growth rates 0.8-10 mg/h are illustrated in fig.2., Mean specific growth rates were determined for {111} faces, the seed orientation being [111]. The spectra were also recorded in the central region of the <111> pyramids.
Fig.1. Concentration of the A and C-centres as a function of growth temperature.
Fig.2. Concentration of the A and C-centres and total amount of nitrogen impurity as a function of average spicific mass growth rate.
# The work has been supported by the Russian Foundation for Basic Research (project 97-05-65195)
59
Fig.3. Concentration of the A and C-centres as a function of total amount of nitrogen impurity.
The character of alteration of the A-to-C-center ratio in diamond crystals as a function of the total concentration of nitrogen impurity is illustrated in fig.3. Herewith the reduced concentration of nitrogen in diamond (N 222) was available by a preannealing of the charge (Ni-Fe-S) in a hydrogen flow at T=1000oC for 1 h. The increased concentration of nitrogen (N 229) was achieved by adding 2 wt% KFeCN6 into the growth system. So , the main factors which govern the preferential formation of the A-centers in diamond in the growth processes are temperature, growth rate, and concentration of nitrogen in the crystallization medium.
The regularities found show that along with the annealing model of formation of complex nitrogen centers in diamond, the growth model earlier proposed [4] is also experimentally confirmed.
With account taken of the obtained data the estimations of the annealing times of natural diamond under the mantle conditions, determined by the relationships between the A,B, and C-centers, do not seem substantiated enough. The complexity of an analysis of the processes of formation of nitrogen centers during the growth of diamond is associated wit the fact that the annealing and growth mechanisms can be operative simultaneously. The regularities found were not revealed earlier possibly because of high rates of the diamond growth which were realized by synthesis of diamond from graphite. Our data suggest that they exceed the rates of the diamond growth, reported here, by 1-2 orders on the average.
References:
Orlov A.I., Khvostantsev L.G. Phase formation in bismuth selenide at high pressure.
key words [ bismuth selenide high pressure]
The most part of bismuth and antimony chalkogenides are minerals. For the problem of the conditions of their genesis in nature to be settled it is important to determine their stability regions at elevated temperatures and pressures.
At normal conditions bismuth selenide as well as bismuth and antimony tellurides have crystal structure of tetradimite type (rhombohedral cell). At temperatures above 500oC and pressures above 2 GPa phase II is stable ( with crystal structure of antimonite type [1,2] - rhombic cell).
In the range from room temperature to 500oC phase II formation is likely to become more complicated. Transformation I—2 begins with essential recession from the equilibrium line and noticeably slows down with decreasing temperature [2,3]. Formation of other bismuth selenide phases was yet observed [3], their stability regions and formation conditions, however, were not determined.
In our study of phase transformations in bismuth selenide we use the technique reported in [4] for bismuth and antimony tellurides. Electroresistance and thermoemf measurements showed that phase transitions were fixed not only with increasing but also with decreasing hydrostatic pressure and temperature. As a result, the P-T diagram shown in the figure was plotted. The equilibrium line for phases I and II at high temperatures (dashed line) was borrowed from [2]. At real experimental conditions, when pressure and temperature change with the velocity of 0.01-0.1 GPa/min and 0.1-1 degree/min, correspondingly, the transformations are realized on achieving the lines marked with arrows. Moreover, at near room temperature, phase II forms through intermediate phase IIa. As it is shown in the figure, transition I—IIa is reversible with some hysteresis and there are P-T regions (filled regions) of the two phases coexistence. In conclusion it should be noted that at pressure above 9 GPa one more metallic phase forms (it was first discovered in [3]). The three above mentioned phases are semiconductors. Our P-T diagram attempts to cover the complex process of phase formation in bismuth selenide in the investigated P-T range.
60
References:
Efimova G.A., Kireenkova S.M. Anomal changes of rocks physical properties at phase transformations under pressure and its relationship with loading conditions.
key words [ calcite phase transformation elastic wave velocity]
The phase transformations in the minerals and rocks are one of the reasons of the tectonic processes in the seismoactive zones. Experiments with the different rocks, minerals, and model materials showed, that anomal behavior of the elastic waves velocities and reducing strength are observered at polymorphic transformation under high pressures. The experiments have been carried out in the piston-cylinder type apparatus at pressures up to 20 GPa and in the hydraulic pressure apparatus under constant hydrostatic pressure with additional axial compression at a constant rate of 1.8 × 10-6 mm/s. The experiments at the conditions of quiasihydrostatic pressure with calcite rocks showed that two phase transformation remarked at 0.4 GPa and 1.6 GPa by change of the elastic waves velocities and attenuation. The texture analysis, conducted by neutron diffraction method showed that the change in sample texture and its turning takes place at the first and second transformations. It was established, that at the first transformation (0.4 GPa) after load was taking off new structural modification of calcite with clear expressional blok orientation appeared. The deformation of marble at a constant hydrostatic pressure of 10 MPa with constant rate showed that a decrease in the elastic waves velocities and the deformational characteristics of sample took place. The breach of the orientation of block structure as a result of transformation has been established by the neutron diffraction method. These results suggest that the processes accompanied by both the breach of the orientation of original block structure and its increasing take place in the earth crust depending on the stress state.
#Fedorov I.I., Sonin V.M., Chepurov A.A., Turkin A.I., Chepurov A.I. The reduction of the silicates and the estimation of their ferriferocity in connection with the diamond genesis.
key words [diamond oxygen fugacity genesis experiment]
According to geothermobarometric data, the main part of natural diamonds were formed under P = 50-60 kbar and T = 900-1400oC [1]. The synthesis of diamond under such P-T parameters can be experimentally achieved only in the presence of the melts of transition metals (Fe, Ni, Co). In case of silicate-, sulphide-carbonic and other systems, the higher P-T parameters are required. This fact suggests that a significant part of natural diamonds had been formed in the presence of native iron, or taenite. The rare occurence of metallic inclusions in natural diamonds, in comparison with those in silicates or sulphides, can be explained by the ability of diamonds self-purification from the previously included metallic phase in the course of their post-crystallization heating in the mantle [2].
The process of the reduction of iron from the silicates and the formation of diamond had been experimentally shown by the authors on the example of the interaction between hydrogene and the fayalite-graphite mixture under P=60 kbars, T = 1500-1550oC. Hydrogene has been obtained in the experiments by the decomposition of TiH2 in the high pressure unit. Fayalite has been decomposed under the influence of hydrogene with the formation of clinoferrosilite, coesite and metallic iron. Due to the catalitic effect of Fe upon graphite, the diamond crystals about 0.2 mm large had been synthesized during the experiments. In the experiments under similar conditions, but without hydrogene the diamonds were absent.
The formation of native iron requires the reductive conditions. We had estimated, that the decomposition of Fe-minerals during the increase of the reductivity of the media (the decrease of fO2) proceeds in such an order: wustite - fayalite - ferrosilite - almandine. For the reduction of iron from the (Fe,Mg,Ca)-solid solutions one requires the higher reductive conditions than for the corresponding reduction of Fe phases. The figure shows the estimated dependence of the ferriferocity XFe=Fe/(Fe+Mg) of olivine, pyroxene, garnet on the fugitivity of the oxygene in the equilibrium with the metallic iron. The value
# The work was supported by the Russian Foundation for Basic Research (Project 95-05-14088, 97-05-65287).
61
of lgfO2 corresponds to the buffer equilibrium fugitivity of the reaction of fayalite, ferrosilite or almandine decomposition. With the growth of temperature, the curves at the figure shift to the area of the lower values of lgfO2, but in principle, the picture does not change.
Fig. The dependence of the olivine (1), pyroxene (2) and garnet (3) ferriferocity on the oxygene fugacity at P=50 kbars, T=1300oC in the equilibrium with the metallic iron.
The olivine and pyroxene inclusions in the diamonds of peridotite paragenesis have the ferriferocity 0.05-0.09 or less, and the garnet inclusions - 0.1-0.2. Clinopyroxenes in the diamonds of the eclogite paragenesis have the ferriferoclty 0.2-0.3, garnets - 0.4-0.7. At the equilibrium with the metallic iron such composition of the silicates corresponds to the values of -lgfO2=10-12 for 1300oC, 50 kbars and -lgfO2= 8-10 for 1500oC, 50 kbars (see fig.). The higher ferriferocity of the inclusions in the peridotite paragenesis probably reflects more reductive conditions of their crystallization in comparison with the eclogites.
References:
Kupin Yu.G. ,* Rusakov V.S. ,* Badyukov D.D. ,** and Kozlov E.A. *** Mössbauer studies of Saratov chondrite subjected to impact superhigh pressure.
key words [iron phase shock melting experiment]
The study of polymorphous transitions and specific features of mineral melting at high pressures is of great significance for understanding the deep structure of the Earth and conditions of its formation, in particular, processes occurred upon the formation and interaction of the mantle with the core. Since it is considered that the core consists of iron metal with admixtures of other elements, and mantle minerals contain Fe atoms, the study of the structural and valent states of iron at P,T-parameters corresponding to the mantle/core boundary is very urgent.
In this work, the states of the iron atoms in minerals of the chondrite meteorite (which is the most probable primary material of planetary bodies) subjected to the impact superhigh pressure were studied by Mössbauer spectroscopy.
A spherical sample (30 mm in diameter) was prepared from a monolithic piece of the Saratov chondrite (L4, fall). The sample was sealed in vacuum in a spherical hermetic cover and loaded with the explosive pressure by a method described in [1]. According to the geometry of impact-wave loading, the pressure and temperature increased toward the sphere center and were estimated by the values of ~300 GPa and ~4000oC in the region remote from the sample center at a distance of ~2 mm. Three main concentric zones appeared in the equatorial plane of the meteorite sample ball: the melting zone (3-5 mm from the center), the zone of pre-melting and strong impact effects (5-8.5 mm from the center), and the zone of retaining the primary chondrite structure with different moderate or weak intense effects of impact metamorphism (8.5-15 mm from the center).
The following iron-containing phases were observed in the probes taken from the regions arranged over the sample radius: olivine, pyroxene, troilite, iron metal, magnetite, and strongly nonstoichiometric wüstite.
A comparative analysis of changes in the relative intensities and hyperfine parameters of the partial spectra gave the following results.
Olivine. The relative content of the iron atoms increases from the external zone to the central one (i.e., as the pressure and temperature increase) and exceeds the initial value by 1.9 + 0.1 times in the melting zone.
Pyroxene. The Fe2+ ions predominantly occupy sites M2 in the orthopyroxene structure in all zones of impact transformations. The melting zone exhibits a strong increase in the heterogeneity in the nearest surrounding of the Fe2+ ions likely related to an increase in disorder in the arrangement of other cations with substantially different ion radii (for example, Ca2+, Al3+, and Na1+).
Troilite. An increase in the number of impurity atoms and the local heterogeneity of the surrounding of the Fe atoms occurs in the melting zone.
Iron metal. A comparatively low amount of iron metal (~12 at.% of the all Fe) was observed for the meteorite sample studied. In the original meteorite, 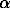 -Fe contains an admixture of Ni atoms. An additional strong enrichment in the Ni atoms is observed in the melting zone.
-Fe contains an admixture of Ni atoms. An additional strong enrichment in the Ni atoms is observed in the melting zone.
62
Magnetite. In the zone of retaining the primary chondrite structure, the magnetite content increases and its structure is formed with a noticeable amount of impurities. This mineral is almost completely absent in the melting and pre-melting zones.
Wastite is characterized by the strong nonstoichiometry and the presence of impurities. An increase in the content of the original wüstite is observed with the approach to the sphere center.
Mutual phase transitions accompanied by redox processes are observed as the degree of the impact action increases. On going from the external zone to the pre-melting zone, iron oxides are mutually transformed according to the scheme: Fe1-xO  Fe1-x+
Fe1-x+ 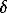 O + Fe3O4
O + Fe3O4  Fe + Fe3O4. A substantial decrease in the amount of nonsilicate iron and a sharp increase in the ferruginosity of olivine occur in the melting zone.
Fe + Fe3O4. A substantial decrease in the amount of nonsilicate iron and a sharp increase in the ferruginosity of olivine occur in the melting zone.
References:
Tomilenko A.A., Chepurov A.I., Shebanin A.P. Fluid inclusions in synthetic diamonds.
key words [synthetic diamond fluid inclusion methane]
Optical and Raman-spectroscopy studies of fluid inclusions have been performed on synthetic diamond crystals produced in the field of their thermodynamic stability but varying growth conditions. Group A diamonds were synthesized at T=1350oC and P=50 kbar in the runs of durations not greater than 2 h, group B diamonds were seed-growth at T=1520-1540oC and p=55-60 kbar, the growth period was from 12 to 30 h.
The plate-like diamond crystals (group A) exhibited predominantly transparent and colorless fluid inclusions. Both unfaceted fluid inclusions, among which there were hollow beaded and disc-like ones, and faceted inclusions (negative diamond crystals) were found. The latters were faceted by the {111} octahedron, {100} cube, {110} rhombododecahedron, and tetragontrioctahedron. The inclusions measured from 5 to 40 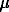 m. All the fluid inclusions were single-phased.
m. All the fluid inclusions were single-phased.
The Raman-spectra of the inclusions demonstrate lines in the spectral subregions of 3000-2700, 1475-1450, 1310-1175, 1150-950 cm-1 (fig.1). The absence of the line in the frequency region of 1600 cm-1 indicates the occurrence of saturated hydrocarbons (alkanes) in the composition of the inclusions. In the frequency region of 3000-2700 cm-1 there appear C-H stretching vibrations localized inside CH2 and CH3 groups. In the narrow Raman spectrum region of 1475-1450 cm-1 there is a line caused by the position of asymmetric bending vibrations of CH3 and shearing vibrations of CH2. CH2 -torsional modes are also manifested in the region of 1310-1175 cm-1. The absence of the lines in the region of 888-837 cm-1 , inherent in nonramified alkanes, suggests that the Raman spectrum has been registered of, most likely, a mixture of ramified and cyclic alkanes wherein this spectral region can be free of lines (Dollish, 1974). Inasmuch as the substance being analyzed in the inclusions is under a high pressure (about 10 kbar), the obtained spectra do not coincide exactly with the available Raman spectra of hydrocarbons (Dollish, 1974) which were usually recorded under normal conditions. According to Seitz et al. the most intensive line (2910 cm-1) of the Raman spectrum of the inclusions in question most likely corresponds to the totally symmetric vibration of the CH4 molecule.
Fig.1 Raman spectra of fluid inclusion in synthetic diamond crystal (group A diamond; solid line) and of the host mineral (dashed line)
Fig.2. Raman spectra of fluid inclusions in synthetic diamond crystal (group B diamond; solid line) and of the host mineral (dashed line)
The synthetic diamond crystals with the octahedral habit plane having secondary {100} and {311} faces (B group) exhibited only dark fluid inclusions with the faceting of negative diamond crystal. They occur as individuals or groups of 2-3 inclusions together with solvent-metal inclusions. The inclusions measure from 5 to 30 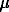 m. The Raman spectra of these inclusions exhibit the lines only in the subspectral regions of 1650-1500 and 3000-2800 cm-1 (fig.2). In the frequency region of 3000-2800 cm-1 wherein the C-H stretching vibrations are manifested only one intensive line of 2910 cm-1 is observed that, according to Seitz et al, (1993), corresponds to the totally
m. The Raman spectra of these inclusions exhibit the lines only in the subspectral regions of 1650-1500 and 3000-2800 cm-1 (fig.2). In the frequency region of 3000-2800 cm-1 wherein the C-H stretching vibrations are manifested only one intensive line of 2910 cm-1 is observed that, according to Seitz et al, (1993), corresponds to the totally
63
symmetric vibration of the CH4 molecule. The line of 1582 cm-1 in the Raman spectrum corresponds to graphite that is likely to film the walls of the inclusions at the inside. The formation of this opaque film is most probably related to the precipitation of carbon (graphite) from the fluid upon cooling. No N2, CO2, and H2 were found in the synthetic diamonds studied by means of the Raman analysis. The Raman spectroscopy identification of H2O and CO2 in the fluid inclusions of diamonds is quite limited. For CO2 this is due to the very strong line of diamond (1331 cm-1) that completely covers the Raman spectrum region characteristic of CO2. For H2O, as shown by Dubessy et al, 1992, the optimal conditions for recording the Raman spectrum of H2O are produced by transforming it to the vapor state which is impossible for the high-density fluid inclusions in diamonds. So, two types of primary (syngrown) fluid inclusions have been found in synthetic diamonds in the field of their thermodynamic stability using optical microscopy and Raman spectroscopy:
(1) -hydrocarbonic inclusions involving, along with methane, considerable amounts of higher molecular than CH4 H-alkanes, and
(2) - essentially methanic inclusions with a graphite film on the inclusion walls.
The inclusions of the first type are characteristic of diamonds synthesized at T=1350oC and P=50 kbar in the runs of which the duration did not exceed two hours, the inclusions of the second type are characteristic of the diamonds grown on a seed at T=1520-1540oC and P=55-60 kbar for 12 to 30 h.
References:
#Kravchenko O.V. , Burdina K.P. , Semenenko K.N. , Matveev A.M. , Tarasova G.M. , and Kulinich S.A. Synthesis and some properties of amorphous carbon nitride C3H4 .
key words [amorphous carbon nitrideDepartment of Chemistry, Moscow State University, Moscow, Russia ]
Interest in carbon nitride C3N4 has recently increased much, because theoreticians have concluded [1, 2] that crystalline carbon nitride with unusual properties can be obtained: it may possess either the negative Poisson coefficient or the hardness greater than that of diamond. However, no bulk crystalline carbon nitride samples have been obtained to date, and all compounds known from published data containing only nitrogen and carbon in a ratio close to C3N4 were obtained in amorphous films with single phenocrysts of the crystalline phase.
Carbon nitride was obtained by the pyrolysis of mercury thiocyanate in different heating regimes. The differential thermal analysis (DTA) shows that under polythermal heating in an argon atmosphere, Hg(SCN)2 at 180oC is rapidly decomposed with a sharp high exoeffect to yield the gas. The isothermal heating also results in the decomposition of Hg(SCN)2 at the temperature close to 180oC after a certain induction period. The decomposition occurs via the following reaction:
2Hg(SCN)2  2HgS + CS2 + C3N4
2HgS + CS2 + C3N4
The composition of the gaseous products of the Hg(SCN)2 pyrolysis was determined on a MI-1201 mass spectrometer. The CS+, CS2+, and S2+ masses were detected in the spectra at 180oC, which are unambiguous evidence for CS2 as the main component. Water and CxHy were determined as admixtures. Mercury sulfide is almost completely removed (HgS is not determined by X-ray analysis) in the 290-300oC temperature range (isothermal heating). However, the density of the powder obtained ranges in different entries: from 2.6 to 2.9 g/cm3. Mercury was determined in the mass spectra.
The treatment of the powder with aqua regia resulted in a decrease in the density to 2.21 g/cm3. The chemical analysis of the amorphous yellowish residue (N-43.13; C-26.4; H- 0.76%) indicates that its composition corresponds to the formula C3N4.2. The CN+, HCN+, N2+, C2N+, and C2N2+ masses were detected in the spectra of this product at 400-480oC, which suggests the decomposition of C3N4 in this temperature range according to the scheme:
2 C3N4(solid)  3C2N2(gas) + N2(gas) (1)
3C2N2(gas) + N2(gas) (1)
C3N4 (solid)  C2N2(gas) + C(solid) + N2(gas) (2)
C2N2(gas) + C(solid) + N2(gas) (2)
We suggested equation (2) because soot was found on the evaporator cover.
Under the action of ammonia at the temperature of 350oC and pressure of 1 kbar, the product obtained is completely transformed into melamine likely in the reaction:
C3N4 (solid)  2NH3 + C3N6H6
2NH3 + C3N6H6
The amorphous C3N4 powders obtained can be well molded and remain unchanged under high pressures (60 kbar) and temperatures below 800oC, while at temperatures >1200oC C3N4 is likely decomposed in part. Graphite and small in volume (20-40 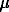 cm) and relative content transparent glassy formations (colorless or light-amber colored) were observed in the quenching samples. According to preliminary data, they are X-ray amorphous.
cm) and relative content transparent glassy formations (colorless or light-amber colored) were observed in the quenching samples. According to preliminary data, they are X-ray amorphous.
References:
# The work was supported by the Program 'Universities of Russia' (Projects #UR-012).
64
#
Surkov N.V., Gartvich Yu.G. Experimental examination of the join pyrope-grossular at pressure 30 kbar.key words [pyrope grossular phase diagram]Joint Institute of Geology, Geophysics, and Mineralogy SB RAS
The experimental data obtained for the join pyrope-grossular are quite contraversal. This particularly refers to the melting region. We have therefore performed an experimental examination of the phase diagram of the join pyrope-grossular at P=30 kb. The work was performed in a high-pressure piston-cylinder apparatus by using a quenching technique (Godovikov A.A., et al., 1971; Boyd, England, 1960; Surkov N.V., 1992). The phase were identified by the X-ray phase analysis and by examining the petrographic microsections, the phase compositions were determined in an electron microanalyser.
The diagram of the join Mg3Al2Si3O12-Ca3Al2Si3O12 at P=30 kb demonstrates the following specific features. Up to T=1075oC the whole of the series of solid solutions in the join pyrope-grossular are stable. Above 1075oC (+25o) clinopyroxene appears in the join pyrope-grossular, and the continuous series of garnet solid solutions has a break.
In the magnesian part of the join the field of the assemblage Gr+Cor+Cpx is located upwards of this temperature to 1370oC. At higher temperatures the assemblage Gr+ Cpx is stable. It melts to Sp+L at T=1525oC. In the calcium part of the join the field of the assemblage Gr+Cor+Cpx is located at temperatures 1075-1170oC. At temperatures in excess of 1170oC it is replaced by the Gr+ Cpx field. Melting of the Gr+ Cpx is observable between 1550 and 1600oC. The higher melting temperatures of this assemblage are likely to be related to the specific features of the sodium chloride cell where hydrated materials are absent.
The compositions of the solid solutions in the phases are in fair conformity with the data of the other authors.
The boundaries of the sold solutions of garnets are nearly coincident with those reported by other experimentalists (Doroshev A.M. et al., 1981; Malinovskii et al., 1983; Boyd, 1970; Sekine T., Wyllie P.J., 1983) with the exception of the data reported by Maaloe and Wyllie, (1979), where the miscibility gap of garnets expands with the decreasing temperature.
Wt.%
Fig.1. Phase diagram of the join pyrope-grossularite at P=30kb. 1.Compositions of the initial mixture. 2- Compositions of the garnet solid solutions. 3- Compositions of the clinopyroxene solid solutions.
The principal difference in the obtained results is that in the magnesian part of the joint the association Gr+Cpx+Cor is stable only below 1370oC and, therefore, corundum is absent on the liquidus, in agreement with the data of Maaloe and Wyllie, (1979) and in disagreement with Malinovskii I.Yu. et al, (1983); Doroshev A.M. et al, (1981).
References:
# The work has been supported by the Russian Foundation for Basic Research project N 96-05-66036
65
petrol. Novosibirsk, pp.32-50.
#
Korochantsev A.V., Badjukov D.D., Moroz L.V., and Pershin S.V. Experiments on impact-induced transformations of asphaltite.key words [asphaltite shock experiment]
Vernadsky Institute, Russian Academy of Sciences, 117975 Moscow, Kosygin St. 19, Russia; e-mail: abasilevsky@glas.apc.org, Institute of Chemical Physics, Russian Academy of Sciences, Chernogolovka, Russia.
Organic materials appear to be present on the surfaces of dark asteroids, some of which are probable parent bodies of carbonaceous chondrites. Asteroids are continuously affected by impacts. To understand the behavior of organic matter during hypervelocity impact, the samples of natural solid bitumen (asphaltite) were shocked to 17.3, 21.3, 23.7, 26.7 and 60 GPa, and its mixture with kamacite - to above 60 GPa and studied by X-ray diffractometry (XRD), IR reflectance spectrometry and C,H,N,S-analysis. Asphaltite was chosen because it spectrally resembles organic matter on some dark asteroids [1, 2].
Two different types of solid fixture were employed for shock recovery experiments: below and above 60 GPa. As a first type, we used plane shock wave experiments for shock-loading up to 60 GPa. The samples were packed into aluminium or copper containers. Shock pressures were generated by impact of a flying plate accelerated by products of detonation to velocities of 1.95, 2.30, 2.50, 2.75, and 3.80 km/c [3]. Peak pressures were calculated using impedance-match methods and the shock-wave equation of state for container material. In a second type of experiments, cylindrical samples were located in steel containers encompassed by charges of high explosive. A geometry of the experiments provided a passage of a disk configuration of shock wave along sample axis at the velocity of detonation of the explosive (above 7.9 km/c) [4]. A pressure distribution along a radius of the sample column is not uniform because of maximum diameter of the shock wave disk is less than the diameter of the sample. Only material located near the central axe of the sample was loaded a single wave. Shock parameters were estimated using experimental [5, 6] and calculated Hugoniot curves. Intensive plastic flow in samples suggests that real post shock temperatures in the samples could be higher than equilibrium post shock temperatures.
XRD patterns (Fig.) allow to trace the dynamics of the structural change in samples shocked at different pressures. After shock-loading up to 26.7GPa, the percentage of ordered aromatics (fa), the average interlayer distance (d002), the average height of the aromatic clusters (Lc), all remained almost unchanged relative to the initial asphaltite structure (fa ~0.25, d002=3.67 Å , Lc ~7 Å ). For the sample shock-loaded at 60 GPa, the percentage of ordered aromatics increased up to about 0.4, and the Lc value - up to 14.5 Å ; the reflection d002 shifted to 3.5 Å . Such parameters are typical of more ordered bitumens - high kerites/low anthraxolites, but not of the initial asphaltites [7]. After shock-loading to above 60GPa, the structure of the sample changed drastically: fa ~0.8, d002=3.40 Å , and Lc=26.5 Å . Furthermore, it revealed a small peak at 3.37 Å corresponding to graphite reflection (002). This much more ordered structure is indicative of still more ordered bitumens - high anthraxolites [7]. Finally, kamacite-asphaltite mixture shock-loaded to above 60 GPa, showed XRD patterns of graphite. The fa value became equal to 1.0. Two types of aromatic ordering were fixed: with d002=3.40 Å , Lc=64.5 Å , and with d002=3.36Å , Lc=141 Å .
FTIR reflectance spectra of asphaltite before and after experiments are shown in Figs. Shock-loading up to 26.7 GPa caused decrease in overall IR reflectance and some loss of aliphatic CH2 and CH3 groups, while the intensi
66
ties of aromatic absorption bands did not change or increase slightly. The samples shocked at 60 GPa show significant drop in overall IR reflectance, further loss of aliphatic groups, complete disappearance of aliphatic chains with C>4, considerable loss of O-bearing groups, and relative increase in intensities of aromatic C-H and C=C fundamentals. In general, the spectra became similar to those of natural high kerites/low anthraxolites [1]. The samples shock-loaded at higher pressures are still darker in the IR region and exhibit completely featureless reflectance curves similar to those of high anthraxolites [1].
The chemical compositions are also consistent with our conclusions. After shock-loading up to 60 GPa the C/(C+H+N+S) (at.) ratio increased from 0.44 (initial asphaltite) to 0.64 typical of high kerites/low anthraxolites. After shock-loading to above 60 GPa the C/(C+H+N+S)(at.) ratio became similar to those of high anthraxolites (0.94).
So, structural, spectral and chemical observations accordingly suggest that shock loading of asphaltite can result in its transformations into other organic species:
The asphaltite shock-loaded up to 27 GPa preserves its initial structure. This is consistent with the earlier results [8], which showed that Murchison (CM2) carbonaceous chondrite shocked to 20 GPa releases little or no of organic matter relative to the unshocked material. After shock loading at 60Gpa, asphaltite transforms into high kerite/low anthraxolite and after shock loading at above 60 GPa - into high anthraxolite. The ordered carbon as graphite appears when asphaltite-kamacite mixture has been shock-loaded to above 60 GPa. This is consistent with complete devolatilization of the Murchison sample occurred at shock pressures above 50 GPa [8].
References:
#
Zhukov A.N. , Burdina K.P. , and Semenenko K.N. The study of the BN-Si system at high pressures and temperatures.key words [BN silicon experiment]
According to the licensed data [1, 2], the use of elemental silicon and its nitride results in a decrease in the pressure of the polymorphous transition 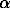 -BN
-BN 
 -BN. According to [3], at 43 kbar the phase transition
-BN. According to [3], at 43 kbar the phase transition 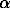 -BN
-BN 
 -BN is not observed; however, boron nitride is dissolved in silicon, and at 67 kbar and temperatures higher than 1450oC
-BN is not observed; however, boron nitride is dissolved in silicon, and at 67 kbar and temperatures higher than 1450oC  -BN is formed. In addition, this work suggests that the formation of
-BN is formed. In addition, this work suggests that the formation of  -BN occurs through the unstable compound Si(BN)x.
-BN occurs through the unstable compound Si(BN)x.
Boron nitride 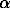 -BN with the content of the main admixture (B2O3) of 0.15% and elemental silicon (semiconductor purity) were used in the experiment. The high-pressure studies were carried out by a standard procedure for the high-pressure technique in "toroid"-type chambers. The samples obtained were analyzed on a DRON-2 diffractometer (CuK
-BN with the content of the main admixture (B2O3) of 0.15% and elemental silicon (semiconductor purity) were used in the experiment. The high-pressure studies were carried out by a standard procedure for the high-pressure technique in "toroid"-type chambers. The samples obtained were analyzed on a DRON-2 diffractometer (CuK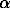 radiation, Ni-filter, Ge-standard).
radiation, Ni-filter, Ge-standard).
The effect of silicon on the 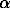

 transition in boron nitride was studied in the pressure range from 50 to 70 kbar at temperatures from 1000 to 1700oC. The field of the
transition in boron nitride was studied in the pressure range from 50 to 70 kbar at temperatures from 1000 to 1700oC. The field of the  -BN formation in the presence of Si is presented in Fig.1. The change in the silicon concentration in the blend in the 3.0-10% range had no noticeable effect on the position of the field of the
-BN formation in the presence of Si is presented in Fig.1. The change in the silicon concentration in the blend in the 3.0-10% range had no noticeable effect on the position of the field of the  -BN formation. It is established that under the P,T-conditions corresponding to the field of the BNsph formation, the reaction products are a mixture of graphite-type and sphalerite boron nitride and a silicon-based solid solution. In the field where no formation of
-BN formation. It is established that under the P,T-conditions corresponding to the field of the BNsph formation, the reaction products are a mixture of graphite-type and sphalerite boron nitride and a silicon-based solid solution. In the field where no formation of  -BN was observed, the samples obtained at temperatures higher than 1100oC consist of
-BN was observed, the samples obtained at temperatures higher than 1100oC consist of 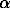 -BN and the silicon-based solid solution. At temperatures lower than 1100oC no formation of solid solutions was observed. The temperature boundary of 1100oC agrees with the melting point of silicon under pressure (1000oC at 60 kbar [4]). It was observed that at 1100oC and 40-60 kbar the cell parameter of the silicon crystal lattice is independent of the treatment time and is equal to 5.429 + 0.003 Å, which corresponds to the cell parameter of pure silicon. A decrease in the period of the silicon crystal lattice was observed at 1400 and 1600oC (see Fig. 2).
-BN and the silicon-based solid solution. At temperatures lower than 1100oC no formation of solid solutions was observed. The temperature boundary of 1100oC agrees with the melting point of silicon under pressure (1000oC at 60 kbar [4]). It was observed that at 1100oC and 40-60 kbar the cell parameter of the silicon crystal lattice is independent of the treatment time and is equal to 5.429 + 0.003 Å, which corresponds to the cell parameter of pure silicon. A decrease in the period of the silicon crystal lattice was observed at 1400 and 1600oC (see Fig. 2).
Fig.1. The region of  -BN formation in the presence of silicon.
-BN formation in the presence of silicon.
# The work was supported by the Program 'Universities of Russia' (Projects #UR-012)
67
Fig.2. Dependence of the parameters of silicon crystal lattice on the time of high temperature-high pressure treatment.
At 1200-1400oC boron nitride is dissolved in silicon, but no formation of  -BN is observed. It can be assumed that the successful recrystallization of boron nitride through liquid silicon to form
-BN is observed. It can be assumed that the successful recrystallization of boron nitride through liquid silicon to form  -BN requires, along with simple dissolvation, the formation of some active complexes" like those mentioned in [3] and that the formation of similar unstable compounds occurs only at temperatures higher than 1400oC. The catalytic activity of silicon is noticeably lower than the action of such substances as Al, Mg, Li, and Ca [5]. The absence of the interaction between boron nitride and silicon to form silicon nitride seems to be most interesting, while the formation of
-BN requires, along with simple dissolvation, the formation of some active complexes" like those mentioned in [3] and that the formation of similar unstable compounds occurs only at temperatures higher than 1400oC. The catalytic activity of silicon is noticeably lower than the action of such substances as Al, Mg, Li, and Ca [5]. The absence of the interaction between boron nitride and silicon to form silicon nitride seems to be most interesting, while the formation of  -BN is preceded by the formation of the corresponding nitride in the BN-M systems, where M = Al, Mg, Li, and Ca.
-BN is preceded by the formation of the corresponding nitride in the BN-M systems, where M = Al, Mg, Li, and Ca.
References:
68