Mineral equilibria in silicate and ore systems
Tolstykh O.N. , Ripinen O.I. The study of the processes of overall crystallization of polymineral associates in flux systems.
key words [mineral synthesis flux]
The interest to the process of overall crystallization is related to the scientific, fundamental importance of the problem and the necessity to develop methods based on real regularities to create commercial technologies, which are very important for a number of branches of national economy.
We have developed the method to obtain polymineral associates of various colours which are analogous to natural precious minerals and with crystals of the same size. The overall crystallization of the phases with the size up
51
to 5 mm was carried out under the conditions of straight (the gradient in saturated melts is 2-5 grad./cm) and inverse (gradient is up to 10 grad./cm) temperature gradient and the lateral source of additional feeding with the use of special regime of temperature decrease. This regime stabilizes the degree of solution-melt saturation that permits to obtain the crystallites similar in size. The multicomponent oxide and oxide-halogen fluxes based on tungstates and molybdates of alkali metals (Li, Na, K), lead-vanadate and bismuth-vanadate systems, Al and Na fluoride systems, borates of alkali metals in various combinations with its fluorides, saturated by the components of natural mineral species analogues were used as a medium of overall crystallization. The growth rate of several individuals is 0,25-0,30 mm per day.
A number of variants were tested as sublayers:
1) natural polycrystalline aggregates, e.g., the samples (plates) of corundum-bearing metamorphic series;
2) the ceramic cakes made of oxides or natural charge in laboratory;
3) factory ceramics of aluminium, magnesium, beryllium and zirconium oxides;
4) spontaneously intergrown crystallites (druses).
The sublayer shape vary, from flat to spherical. The keeping time in the unit for polycrystalline aggregates synthesis is from two to three weeks.
Thus, we have obtained the following associates of synthetic minerals: crimson-colored ruby - green-violet alexandrite; pink rhodonite - bustamite (Mn,Ca)3[Si3O9], pink spinel- red taaffeite; green beryl-blue fenacite, light green lavrovite-brown enstatite, gold-yellow zircon-blue phenacite; emerald-green tsavorite (grossular). The druses with the combination of three mineral species, i.e. chrysoberyl, phenacite and emerald, were obtained during the solution and subsequent overall crystallization of beryl.
It should be noted that different combinations of mineral species with different addition of aluminium and beryllium oxides at the output of experiments in the common solution-melt system from the beryl charge of Malyshev deposit have been obtained, i.e. when the beryl structure is broken up, lnetphenacite-alexandrite, mullite-chrysoberyl, phenacite-mullite and tridymite-phenacite-alexandrite, etc. are produced, the relation of the above phases could be varied with the help of additives precisely. Thus, when adding 1 mole of beryl to 4 moles of aluminium oxide and 14 moles of beryl oxide, we'll obtain chrysoberyl and phenacite intergrowths in equal proportion at the output. On addition of 8 moles of Al2O3 and 18 moles of BeO, the proportion of chrysoberyl becomes greater (its area is about 1,5 times greater than phenacite one). On BeO addition only phenacite prevails (its area is 6 times greater that chrysoberyl one at the output). Thus, different variations of phases at the output could be obtained changing the proportion of beryl, Al and Be oxides additives at the input. Colour variations are observed respectively.
The use of heteroepitaxy, i.e. mineral intergrowth with different crystalline structures is another not less interesting aspect. We noticed, that chrysoberyl (alexandrite) (orthorhombic system, rhombopyramidal symmetry) greatly grows over the matrix of natural corundum (ditrigonal-scalenohedral symmetry). Ruby and alexandrite intergrowths are formed making at the output the excess of Al oxide in the charge. Similar phenomenon is observed in the composition of beryl and topaz (emerald and topaz druses) and spinel and taaffeite as well. It turns out that under certain conditions the minerals of various types of symmetry are isostructural, i.e., the lattices coincide in parameters. This property could be used at the growth of one mineral monocrystals over the seed of the other one, it is only significant to find the direction of matrix plane for optimal growth of other mineral.
On the colour and morphology of individuals. It is very simple to select the colours of synthetic minerals to make them similar to the natural mineral with the help of chromophore ions (Cr,Fe,Mn,V,Ti,etc.). Let us take ruby as an example. We can obtain the synthetic ruby similar to the natural one (e.g., of Birma and Ceylon), but the crystals are to be flat (foliated or flaky). It should be noted that non-chromophore ions of gallium, scandium influence both the colour and morphology of crystals. The crystals generated are three-dimensional and with corresponding colour tint (pigeon blood colour, i.e., the combination of red and purple), being just the same as it is observed in natural objects. In this case the concentrations of the non-chromophore ions are some tens-hundreds of percent fractions.
Conclusions:
1) The flux polycomponent systems during the selection of optimal parameters of growth permit to obtain various polymineral associates (associations), which make it possible to model analogues of natural mineral species.
2) Both natural and synthetic objects could be used as sublayers to obtain druses and polymineral associate druses.
3) Breaking up the natural minerals of complex chemical composition (beryl, garnet, etc.) we can get various proportions of synthetic analogues of elementary composition in the flux systems with the help of additives (oxides). The process is defined by the number of analogues and the regime of overall crystallization.
4) The use of heteroepitaxy in the experiment can solve the problem with seed or sublayer, since in certain directions the minerals of different crystalline lattice are isostructural.
5) The non-chromophore ions play an important role in the change both in morphology and colour characteristics of synthesized phases during the crystal growth. Their small amounts (fractions of percent) can improve both the morphology and colour.
Tolstykh O.N. Experimental investigations of mineral synthesis with the help of metallothermic processes.
key words [metallothermy mineral synthesis]
52
Metallothermy as a special direction is increasingly employed in different fields, including search experimental mineralogy. It is traditionally used to obtain ferric fusions, production of rare refractory metals, special fusions, simple and complex inorganic compounds. Our five year experience has shown the wide potential of using metallothermic reactions for deep complex working of mineral sources to extract precious and rare metals.
The use of metallothermic process heat permits to give up heaters and to proceed crucibleless heating, thus making the method more simple. This process is absolutely harmless from the point of view of ecology. The above facts seem to be the doubtless advantage of metallothermy against other methods.
The metallothermic method has attracted our attention as a method to obtain analogues of natural mineral types. The high-energetic reactions with peculiar powerful heat release, simple and complete separation of the initial compound from the obtained product are used as a basis of this method. Owing to the strong exothermical effect (the temperature reaches 3000oC and higher) of the metallothermic processes reactions, the fusion of the total mass of the charge occurs. Finally a metal ingot (bead) and a crystallized 'slaggy phase' are obtained. Regimes of optimal speed of cooling (to obtain macrocrystalline mineral mass) and crystallization are worked out by selecting the flux additives that, in fact, regulate the speed of the reaction.
In fact, the metallothermic process is carried out within the self-forming crucible (so-called autocrucible) and accordingly the peripheral part is more microcrystalline, than the central one. It is worth saying that the crystallinity (blocking) degree of the synthesized mineral substance depends on the mass of initial charge. Our standard samples were 50-100 g, so monophases up to 5 mm were obtained. These dimensions permitted to make different research operations (photo-survey of optical spectra, followed by the calculations of colour characteristics, R and IR-spectroscopy, x-ray spectral microanalysis, etc.). Depending on the composition of the initial charge, an optimal complex of reagents is selected to perform the smooth process (not explosive). The reaction takes 5-7 minutes and is followed by natural cooling in the open air. According to the stability field of the synthesized phase, we have tried to perform metallothermic reactions both in vacuum and at high initial pressures (up to 15 kbars).
Thus, during the reaction of alumino-thermic reduction, corundum will be a slag-forming product, and when we intrude chromophore into the charge, a wide range of colours from red (ruby) to dark blue (saphire) can be obtained.
The use of metallic magnesium together with aluminium in the initial charge allows to get differently coloured spinels (MgAl2O4). The selection of the flux additives greatly influences the speed of metallothermic reactions and crystallization speed accordingly. The following compounds and their combinations such as fluorite, borax, cryolite, halite, sylvite, villiaumite, etc. were tested as solution-melt systems. All these compounds must be dried up, as even a small fraction of a percent of moisture can cause explosion.
Compositional variations of the initial charge have given a possibility to synthesize many other minerals, such as rutile (TiO2), baddeleyite (fianite) (ZrO2), gahnite (ZnAl2O4), heterolith (ZnMn2O4) and many other analogues of natural mineral types.
The realization of diamond synthesis and growth is supposed to be the most interesting use of metallothermic reactions. The metallothermy with carbon-bearing compositions under isochoric conditions with the initial pressure of about 12-15 kbars in the chamber allows to reach the diamond stability field through the raise of temperature to 2500-30000C. The initial intrusion of diamond powder of 5-10 microns into the charge followed by metallothermic reactions permitted to get diamond crystals up to 500 microns in size.
Thus, we evaluate the use of metallothermic reactions in mineral phase synthesis as a long-term trend in different fields. The new methodical approach of investigations of the mineral formation in dry systems is presented from the fundamental point of view.
Tolstykh O.N. Experimental methods for determination of metals concentration and for highly efficient processing of gold-bearing concentrates.
key words [gold extraction ore]
The variety of the mineral composition of phase-associates of gold and other seven precious metals is due to specific type of the deposit. They may be represented by oxides (elementary and composite), sulphides, silicates, phosphates, tungstates, sulphates, etc. Both in alluvial placers and in primary deposit.
The aim of this investigation is the greatest extraction of gold from different types of mineral samples with the minimum of energy and time expenditure and with no ecological hazards at the processing of raw material.
The operating technological processes to extract precious metal involve great harmful conditions of production (cyanidation, hydrochlorination, electrolyte refining, amalgamation, etc.). They require a considerable energy consumption and are rather low efficient both with respect to the complete extraction of precious metals and to time expenditure.
The proposed procedure provides for several methods of enrichment, based on the processes of chemical decomposition (change into water-soluble form), melt-saline method (extraction of non-ferrous and ferrous metals) and metallothermic method (extraction of precious metals into collector and separation of "non-precious metals" into slags). The use of each of the above listed methods depends on the specific of raw material. In this connection mineragraphic investigation (mineral composition analysis) and thorough test by the method of express-analysis in situ should be essential before the use of the method.
53
The principle of the analytical express method is that a vigorous reducing agent is employed to transfer precious metals from intermediate product to collector (copper, nickel, iron, tin, chrome, etc.). The proportion of components products containing precious metals, metal reducing agent, collector and fluxing agent varies depending on the initial charge. The basis of this techinque is an equipment which comprises dielectric filler, launcher, gas outlet (it highly volatile sulphur, selenium, tellurium and arsenic are present) and mechanical decantation of precious metal. To obtain a refined metal the equipment of electrochemical decomposition of the metals and furnace for finishing melting (refining) are used. The probe of the final product is controlled by ² Gold Tester² apparatus, made by RS MIZAR firm (USA). As a rule, the probe of the final product is 99,9%. Weighing and conversion to the corresponding unit sample are performed.
Thus, we have approved the synthetic concentrates, i.e. the mixture of real samples and thin gold powder of 583 probe artificially inserted with the variations of gold content in concentrate from 0.1 to 60 wt.%. The degree of gold extraction is 99,99%.
To determinate the concentration of the gold up to 10-6 wt.% it is necessary to use 5-100 g. of the sample for analysis. The time expenditure is 3-5 hours, and tens samples can be analyzed simultaneously.
The given method seems promissing for the concentration during metal mining both in primary deposits and in placers.
Glazkova M.A. , Khramov D.A. , Bychkov A.M. , and Urusov V.S. Distortions of the tin-containing ilmenite structure at different oxidation states.
key words [tin containing ilmenite]
V. I. Vernadskii Institute of Geochemistry and Analytical Chemistry, Moscow, Russia
It is known that ilmenite and hematite at T» 1000oC form continuous series of solid solutions (partially oxidized ilmenite is commonly named ferriilmenite), and the Fe3+/Fe2+ ratio is an indicator of the oxygen fugacity at the depth of the mineral formation. M÷ssbauer spectroscopy (MS) is a direct method for determining this ratio. In addition, ferriilmenite under natural conditions is a concentrator of tin. Therefore, problems on the solubility limits of tin and its valent state and structural site in ferriilmenite are of substantial interest.
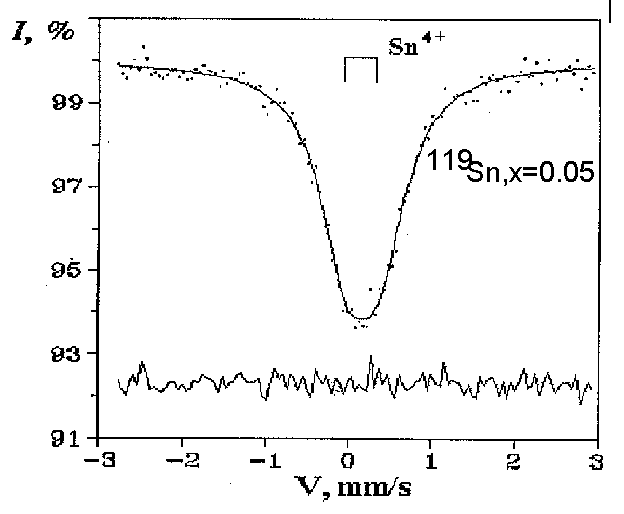
In the present work, samples of the FeTi1-xSnxO3 (x = 0.01; 0.05; 0.13; and 0.22) system were synthesized by annealing (T = 1150oC, t = 45 h) of the starting components in evacuated quartz ampules followed by quenching in air and were studied by MS of 57Fe and 119Sn nuclei and X-ray phase analysis (XPA).
MS 119Sn. The samples with x = 0.01 contain tin only in the tetravalent state, while both tetra- and bivalent tin is present in the samples with x = 0.13 and 0.22 (Fig. 1). The values of isomeric shifts and quadruple splitting indicate that the Sn4+ ions are localized in the ilmenite structure. Only the cases of localization of the Sn2+ ions in the near-surface layers of crystallites of transition metal oxides are known from published data. However, in our case, such factors as the absence of oxidation of bivalent tin in air during a long time and the character of the dependence of the unit cell parameters of ferriilmenite on the number of the Sn4+ and Sn2+ ions suggest that the latter are located in the structure. Taking into account the aforesaid, under the given conditions of synthesis, the solubility limit of tin in ferriilmenite was 22(2) at.%.
MS 57Fe. The analysis of the spectra showed that all samples contained both bi- and trivalent iron. The Fe2+ ions by the isomeric shift value can be divided into two groups: with d1~1.1 mm/s and d2~ 0.9 mm/s, which is characteristic of the octahedral and tetrahedral environments of bivalent iron by the oxygen ions, respectively. However, it is known that cations in the ilmenite structure occupy only octahedral sites. The following model can be supposed to explain this fact. The Fe3+ ions in the ilmenite structure are known to form hematite-like clusters. The presence of such clusters should lead to strong distortions of the structure appeared as the compression of the unit cell along axis c (which is confirmed by the XPA data), since the parameter c = 13.7483 ┼ in hematite is substan-
54
tially lower than c = 14.0855┼ for nonoxidized ilmenite. Fig.2 shows how the compression of the Fe3+O6 octahedron along axis c results in the deformation of the adjacent F2+O6 octahedron. In this case, the coordination of the Fe2+ ions from the viewpoint of comparison of the metal-oxygen bond lengths can be considered as "pseudotetrahedral," which explains the anomalously low value of d2. Based on the aforesaid, we can suggest the following crystal chemical formulas for the tin-containing ferriilmenite samples:
(Fe2+0.95Fe3+0.15Ti4+0.85Sn4+0.05)O3
(Fe2+0.86Fe3+0.24Ti4+0.77Sn4+0.07Sn2+0.06)O3
(Fe2+0.81Fe3+0.29Ti4+0.68Sn4+0.13 Sn2+0.09)O3
# Kravchenko T.A. and Kolonin G.R. Dependences of stable forms of platinum and palladium concretions on the composition of copper-containing sulfide associates (by experimental data)
key words [platinum palladium sulphide experiment]
Elevated content of Pt and Pd are known to be abundant not only in copper-nickel, but also in copper-pyrite sulfide deposits [1, 2]. The results presented in this work supplement and extend substantially the preliminary data presented earlier in [3] and should be fruitful for studying the mineral composition of these deposits.
Experiments were carried out using the ampule method by crystallization of samples from the sulfide melt followed by annealing at 600oC. The starting weighed samples were related to the central part of the triangle of the Cu-Fe-S compositions. The majority of the chosen points corresponded to join of 45 and 50 at.% S and were adjacent to the stability region of an intermediate solid solution (Iss), which gives chalcopyrite and phases close in composition due to subsolidus transformations below 600oC. Platinum and palladium were introduced to the weighed sample separately or together in the amount of 1 wt.%. The fugacity of gaseous sulfur (fS2) was measured during annealing of samples by the pyrrhotite method [4]. The phase composition was determined by X-ray microspectral analysis.
The main results are presented in table and show that the formed platinum and palladium forms change from intermetallic to sulfide forms as the fugacity of sulfur and the Cu : Fe ratio increase in accordance with a change in the composition of the main sulfide minerals. A similar situation was observed for Pt in the Me9S8 join of the Cu-Fe-Ni-S system [5]. When 13 > -logfs2 > 6, Pt is crystallized in the form of isoferroplatinum Pt3Fe, and Pd is characterized by the Pd-analog of tulaminite Pd(Cu, Fe). When logfS2 = -6, the Pd-analog of isoferroplatinum Pt3Fe is formed. The alternation of the concretion forms from intermetallides to sulfides occurs at 6 > -logfS2 > 5, and combined crystallization of Pt3Fe and PtS-cuperite is observed for Pt, while Pd on cooling from the melt forms sulfide (Pd, Cu)16S7 with 53 at.% Pd, which decomposes at 600oC to form (Cu, Pd, Fe)3S with 20 at.% Pd and incorporation of a portion of Pd into Bnss. The further increase in the fugacity of sulfur (-logfS2 < 6) is accompanied by the formation of sulfides only: cuperite and malanite Cu(Pt, Fe)2S4 for Pt and vysotskite PdS for Pd.
When Pt and Pd are crystallized in combination in the cooling regime, the same phases are formed from the melt as for their separate participation in experiments. They are characterized by the following specific features: (1) formation of complicated grains, whose central parts are formed by platinum phases (always with Pd), and peripheral parts are formed by palladium phases (with or without Pt); (2) enrichment in iron of the platinum phases and in copper of the palladium phases. The composition of the complicated grains is redistributed during annealing, and the boundaries are smeared up to complete disappearance. The associations with free copper (upper lines in the table) exhibit a tendency of the (Pt, Pd)(Cu, Fe) compounds to be formed with the equal ratios (25 at.%) of Pt and Pd. The associations with Iss at 9 > -logfS2 > 6 are characterized by incorporation of Pd into Pt3Fe and surrounding sulfides, which increases as the sulfur fugacity increases. Braggite (Pt, Pd)S containing 25 at.% Pt and 25 at.% Pd is formed in the region of cuperite and vysotskite crystallization (bottom part of the table).
# The work was financially supported by the Russian Foundation for Basic Research, (project N 96-05-66036)
55
Table 1. Forms of Pt and Pd concretions in the Cu-Fe-S system at 600oC.
|
Association of main sulfides |
Composition, at.% |
-lgfS2 |
EPG content of the phase |
||||
|
Cu |
Fe |
S |
Pt |
Pd |
Pt+Pd |
||
|
Bnss+Po+Cu |
22-15 |
33-40 |
45 |
13-10 |
Pt3Fe |
Pd(Cu,Fe) |
(Pt,Pd)(Cu,Fe) |
|
Bnss+Po+Cu |
40 |
20 |
40 |
10 |
Pt3F |
Pd(Cu,Fe) |
(Pt,Pd)(Cu,Fe) |
|
Bnss+Iss+Po |
27-25 |
28-30 |
45 |
9-8 |
Pt3F |
Pd(Cu,Fe) |
Pt3Fe (up to 3%Pd) |
|
Bnss+Iss |
30-27 |
25-28 |
45 |
8-7 |
Pt3F |
Pd(Cu,Fe) |
Pt3Fe (up to3%Pd) |
|
Iss+Po |
10 |
40 |
50 |
6 |
Pt3F |
Pd3Fe |
Pd3Fe (up to5 % |
|
Bnss+Iss |
33 |
22 |
45 |
6-5 |
Pt3Fe |
Bnss (up to 3% Pd) |
Pt3Fe (up to8%Pd) |
|
Iss+Po |
18-15 |
32-35 |
50 |
same |
PtS |
PdS |
(Pt,Pd)S |
|
Iss |
22-20 |
28-30 |
50 |
4-3 |
same |
same |
same |
|
Bnss |
56 |
4 |
40 |
>3 |
Cu(Pt,Fe)2S4 |
||
Note: Bnss is the bornite solid solution; Po is pyrrhotine; Iss is the intermediate solid solution (Cu, Fe)S2, and Cu is metallic copper.
References:
Mel'chakova L.V. , Kiseleva I.A. , and Ogorodova L.P. Phase transformations in natural and modified zeolites (by calorimetric data).
key words[phase transformation zeolite]Department of Geology, Moscow State University, Moscow, 119899 Russia mineral@geol.msu.ru
Calorimetric studies allow one to detect phase transformations on DTA curves and on measuring heat capacities and to determine the energy of these transformations. Phase transitions in zeolites are mainly related to transformations in the alumosilicate framework of both H2O-containing and dehydrated zeolites.
We studied natural samples of fibrous zeolites, zeolites of the analcime, mordenite, and heulandite groups, and the cation-substituted (K-, Tl-, Cs-, NH4-, Ag-, Li-, and Rb-) forms of natrolite obtained from the natural natrolite sample by cation exchange in molten salts of the corresponding cations.
Calorimetric studies were performed within the -170 to +700oC range on a Mettler TA-2000B differential scanning calorimeter (DSC) (Switzerland) and a Setaram heat conducting high-temperature Calvert microcalorimeter (France).
A reversible phase transition in Li-zeolite (bikitaite) was observed for the first time in the low-temperature region. The transition was detected on the heating curves (DTA) and on measuring the heat capacity. The transformation occurs in a narrow temperature range (about 10o) with Tmax = -53oC. The maximum excessive heat capacity of the transition was 24.5 J/mol × K for the Li2[Al2Si4O12] × 2H2O composition. The transformation observed is close to the second-order phase transition.
The measurements of the heat capacity in thomsonite, gonnardite, and edingtonite established small anomalies in the low-temperature range (from -153 to -63oC). The phase transition in edingtonite has been previously detected by adiabatic calorimetry, dilatometry, and NMR spectroscopy and explained by proton disordering [1]. The anomalies in the behavior of the heat capacities of thomsonite and gonnardite likely have the same nature.
Reversible phase transformations in the cation-substituted forms of natroline were observed by the DSC method on the DTA curves recorded in the cooling regime: in K-natrolite with Tmax = -25.7oC and the enthalpy of transition of 0.65 kJ/mol and in Tl-natrolite with Tmax = -29.6oC and the enthalpy of transition of 5.3 kJ/mol. As shown in [2], these transitions are related to changes in the alumosilicate framework and characterized by a decrease in the volume.
The calorimetric studies in the temperature range higher than room temperature observed a reversible phase transformation in vairakite in the 80-170oC range with a
56
pronounced maximum at 147oC. The transition was detected by measuring the heat capacity and in the DTA curve obtained by the DSC method. The enthalpy of the phase transition was measured: 1.70 +0.04 kJ per mole of CaAl2Si4O12*2H2O. This transformation close to the second-order phase transition was observed and studied by other methods in [3]. It is related to a change in the structure of the zeolite framework and positions of water molecules [3].
The phase transition in metanatrolite (dehydrated natrolite) was studied on a Calvert microcalorimeter in the 384-696oC range by the "discharge" method. A jump of the enthalpy increase and a change in the slope of the temperature dependence of H0T-H0298.15 were observed at 563+3oC. This can be related to the phase 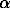 -
-  transformation in metanatrolite described in [4] at a temperature by 50o lower. The enthalpy of the phase transition 14 +- 10.0 kJ per mole of Na2Al2Si3O10 was calculated from the experimental data.
transformation in metanatrolite described in [4] at a temperature by 50o lower. The enthalpy of the phase transition 14 +- 10.0 kJ per mole of Na2Al2Si3O10 was calculated from the experimental data.
References:
# Sinyakova E.F., Shestakov V.A., Kosyakov V.I. Liquidus surface of the Fe-Nis system at xs<0.51.
key words [iron nickel sulphide melt experiment]
The Cu-Fe-Ni-S system is of importance for the petrology of sulfide Cu-Ni ores. The modeling of the crystallization processes in this system necessitates the information on the conditions of phase equilibria with the participation of a sulfide melt. Presently such data are scarce not only for the quaternary system but for the ternary systems, which faceting it, as well. In this work we have studied a portion of the liquidus surface of one of such systems, Fe-Ni-S, in the sulfur-depleted region. According to [1], a monosulfide solid solution (FexNi1-x)S1+y (mss), a 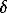 -Fe-base solid solution, a Fe-Ni solid solution with the structure of
-Fe-base solid solution, a Fe-Ni solid solution with the structure of 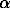 -Fe (tenite-tn), a heazlewoodite solid solution (FexNi1-x)S3+yS2 (hzss). There are two versions of Ni-S phase diagram. According to [2] a hzss does not get exsoluted and is a single phase. According to [3] there are two solid solutions with a narrow two-phase region separating them. The resolving power of the traditional methods is not capable of distinguishing between them, therefore, we have taken the simplest version of the work [2]. The cited data suggest that the liquidus surface consists of four fields of primary crystallization.
-Fe (tenite-tn), a heazlewoodite solid solution (FexNi1-x)S3+yS2 (hzss). There are two versions of Ni-S phase diagram. According to [2] a hzss does not get exsoluted and is a single phase. According to [3] there are two solid solutions with a narrow two-phase region separating them. The resolving power of the traditional methods is not capable of distinguishing between them, therefore, we have taken the simplest version of the work [2]. The cited data suggest that the liquidus surface consists of four fields of primary crystallization.
The published data concerned with the liquidus of the system in question are not numerous. In [4-6] the cross sections of the phase diagram were plotted at 725, 900, 1000, and 1100oC. The sections for xs =0.47 and Fe/Ni=1 were plotted fully or in part in [7]. Some quantitative data on the liquidus region adjacent to tenite were reported in [8]. Here we have studied a portion of the liquidus surface in the range of 37-51 at % S using DTA, X-ray phase analysis (XPA) , and optical microscopy (OM). The samples were synthesized from grade A-2 carbonyl iron of special purity, H-1y nickel, and special-purity sulfur. The liquidus temperatures were defined from heating-cooling curves of 20-30 mg samples inserted into evacuated quartz capsules. The typical rate of the temperature variation was 20 deg/h. The accuracy of the temperature determination for the effects on reference samples was +5oC on the average. The real accuracy of the liquidus temperature determination was much lower because of the errors in the sample preparation, possible interaction of some samples with the quartz glass, and low rates of the solid-state diffusion. The cooled and quenched samples were examined by methods of XPA and OM in order to determine to what field of primary crystallization the point on the liquidus surface having the composition identical to that of the studied sample can be assigned. Certain difficulties, encountered in solving this problem, are associated with the occurrence of several nonquenchable solid solutions forming, upon cooling, small, complex in the phase composition, aggregates which are difficult to recognize. It has been found that the compositions in question are related to the fields of primary crystallization of mss, hzss, and tn. The LSM was used to construct polynomial approximations describing the corresponding portions of the liquidus surface. The available data on the liquidus of the faceting binary Fe-Ni, Fe-S, and Ni-S systems, and the results of [8] were taken into account in constructing the surface. In order to determine the boundaries of the primary crystallization fields the lines of intersection of the obtained surface were found. The experimental data on the crystallization field of the 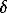 -phase were confined to information on segments of liquidus lines of the Fe-Ni and Fe-S binary systems. Using this information, we have approximated the
-phase were confined to information on segments of liquidus lines of the Fe-Ni and Fe-S binary systems. Using this information, we have approximated the 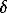 -phase field by the linear surface. Then using the obtained equations of the liquidus surface portions we have plotted isotherms.
-phase field by the linear surface. Then using the obtained equations of the liquidus surface portions we have plotted isotherms.
The obtained fusibility diagram is illustrated in the figure. The AB line corresponds to the peritectic reaction 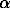 +
+  = tn. The CDE line connects the binary eutectics of the Fe-S and Ni-S systems, and the DFH line corresponds to the peritectic reaction of the hzss formation.
= tn. The CDE line connects the binary eutectics of the Fe-S and Ni-S systems, and the DFH line corresponds to the peritectic reaction of the hzss formation.
# The work has been supported by the Russian Foundation for Basic Research (grant 96-05-66036)
57
References:
58