Isotopy
Spasennikh M.Yu. Transport and fractionation of isotopes at a phase convection in the melt-solid phase system.
key words [phase convection isotope fractionation]
Vernadsky Institute of Geochemistry and Analytical Chemistry, RAS Moscow
It is general knowledge that the constants of isotopes equilibrium fractionation decrease with the growing temperature, becoming very small at temperatures characteristic of magmatic processes [1]. It is therefore believed that fractionation of isotopes between a melt and minerals cannot give rise to considerable isotopic variations of magmatic rocks [2]. This point of view is, however, not always valid. As will be shown in what follows under appropriate conditions in a magmatic chamber there can occur a significant spatial differentiation of the isotopic composition. This situation is possible if the connective substance transfer processes proceed in a specific manner. In particular, favourable conditions take place at phase convection in the melt-solid phase system.
The phase convection is considered to mean the process of free heat convection in a multiphase system when the moving velocities of different phases are unlike. A particular case of this process is sedimentation of mineral crystals in a melt. The role played by the phase convection in the formation of mineralogic zoning of magmatic rocks was considered in the works [3,4].
This work deals with an analysis of the mathematical model that describes transfer and fractionation of isotopes at the phase convection. The model is based on the system of differential equations in partial derivatives. The situation is considered where sedimentation of mineral particles in the magmatic chamber is accompanied by the isotopic exchange with the melt forced upward (fig.1). Mineral crystals are assumed to form near the upper boundary of the chamber, and their melting or accumulation takes place in the lower system's part. Taking oxygen isotopes
74
as an example, it has been shown that under such conditions there can arise a considerable vertical gradient of the isotopic composition of the melt and the solid phase. The ultimate gradient value on the vertical coordinate 2 is
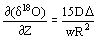
where w is the crystal sedimentation rate, R is the linear crystal size, D is the coefficient of the interdiffusion of oxygen isotopes in the crystal, D is the magnitude of isotopic fractionation between the crystal and the melt.
Fig.1. Schematic presentation of circulation of the substance in a cell with phase convection.
The analysis has shown that the most favourable conditions for the isotopic differentiation are realised in the case of melts of basic composition having low viscosity. For magmatic chambers of hundreds-of-meters vertical size the difference in the isotopic composition of the substance between the lower and upper boundaries can exceed the D value by tens and hundreds of times, in this case the upper part of the section is depleted in isotope 18O compared with the lower part. The time periods over which a considerable inhomogeneity of the isotopic composition arises in the course of the phase convection are comparable with the time periods of solidification of large intrusives. The process of occurrence of isotopic inhomogeneity at phase convection in the system 500m in height is shown in fig.2.
The parameters of the problem are: w=12.3m/y, R=0.3 mm, D=10-15 m2/s [5]. The analysis of the geochemical literature shows that many geological subjects exhibit depletion in 18O of the upper part of the section. In many cases it has been proved that such zonings are the result of the action on the rocks of waters of oceanic or meteoric genesis. There are, however, situations [2,6] where such explanation is not supported by geochemical data. For example, in magmatic rocks strongly depleted in 18O secondary mineral phases are often nonpresent which should have formed at the interaction of rocks with a great amount of waters of the crustal genesis. Possibly, in these cases zonings can be related to the processes considered in this work.
Fig.2. Partitioning of the isotopic composition of solid oxygen in the cell with phase convection illustrated in fig.1. The curves corresponding to different instants of time elapsed since the onset of the circulation. Line 1 is the starting isotopic composition of the solid phase. Curves 2,3,4,5,6,7, correspond to 240, 480, 960, 1440, 1920, 2400 years. Line 8 is the steady-state of the system achievable over about 104 years.
The indications of the effect of the phase convection on the isotopic composition might be sought in the phenomena of planetary scale. Once the vertical size of the system grows, the magnitude of the possible differentiation also increases.
If such processes are occurring (or occurred earlier) under the lower crust-upper mantle conditions of the Earth, they can well be one of the reasons for the existing considerable inhomogeneity of the isotopic composition of the crust and the mantle existing for different elements.
References:
75