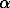 -quartz on
-quartz on  -quartz), on crystal dimensions and their habit planes.
-quartz), on crystal dimensions and their habit planes.VIII. Synthesis, properties and modification of minerals
(Leader Prof. V.S.Balitsky)#Balitsky V.S., Balitskaya L.V. Experimental modeling of concurrent processes of dissolution and growth of mineral single crystals in supercritical water fluids.
An experimental modeling has been performed for concurrent processes of spatially integrated and spatially disintegrated dissolution and growth of some mineral crystals characteristic of pneumatolytic-hydrothermal mineral formation stage (quartz, corundum, tourmaline, topaz, and fluorite). More than 300 runs (10 to 60 days long) were performed at temperatures from 400 to 900oC and pressures from 0.3 to 1.5 kbar using heat-resistant autoclaves and Tuttle bombs designed in the IEM RAS. Mineralizers were pure water, alkali, neutral , and acid water solutions of alkali-metal chlorides and fluorides, and boric acid.
The physicochemical conditions have been found at which spatially integrated and disintegrated processes of dissolution and growth of quartz and topaz crystals can concurrently occur in direct thermogradient field. This phenomenon is related with likeness or unlikeness in signs of their solubility temperature coefficients fluids of various compositions and, also, with fluid density, and kinetics of dissolution and growth of quartz and nucleation of topaz phase.
Once corundum is interacting with acidic fluoride fluids, irrespective of their density (0.1-0.7 g/cm3 interval), the alumina transfer is accomplished in the direction opposite to direct temperature gradient. This fact attests the generality of thew alumina transfer phenomena at recrystallization of corundum and topaz, although an intensive dissolution and growth of the latter occurs only in the presence of quartz. In the case of joint occurrence of quartz and corundum in fluids of said composition the both minerals are replaced by topaz or x-andalusite (depending on the fluorine concentration in the fluids). In alkali fluids of moderate density (0.4-0.6 g/cm3) alumina, at temperatures 500-700oC, is characterized, like silica, by a direct transfer. At a joint occurrence of quartz and corundum, in strong-alkali fluids under thermogradient conditions the both minerals are replaced by feldspars.
Fluorite in acid fluoride fluids is practically undissolvable. It retains stability in the presence of quartz and topaz too, although the latters undergo herewith an intensive redeposition, as it takes place in the absence of fluorite.
Tourmaline behaves extremely inert an acid water-borate fluids (concentration of H3BO3 is less than 6 wt%). As the H3BO3 concentration grows to 12 wt%, both the seed and charge tourmaline crystals are replaced by a thin schorl film (possibly, by diffusion, due to iron coming into the solution at corrosion of the autoclave walls). In this case regularly faceted small (fractions of mm) schorl crystallites form on the surface of the seed crystal, and the charge crystal exhibits traces of dissolution. The presence of quartz is of no effect on the character of dissolution and transfer of tourmaline the same as the latter is of no effect on the intensity of dissolution and redeposition of quartz. In alumofluoride acid fluids tourmaline is unstable, it dissolves intensively with the formation of at least two new phases. Additional introduction of quartz into the system does not change the incongruent dissolution character of tourmaline. The occurrence of tourmaline in these fluids does not affect the dissolution and growth of quartz crystals as well.
Using the interaction of said minerals with water fluids of various compositions as examples, it has been shown for the first time that in the process of their dissolution and redeposition under the conditions of free temperature convection between dissolution and crystallization regions there always arises a narrow transitional (equilibrium) zone free of any signs of dissolution and growth. The position of this zone in space and time is dictated both by the volume and total surface of dissolving minerals and by the magnitude and character of temperature gradient alteration.
##Bublikova T.M., Balitskii V.S., Mar'ina E.A. Specific features of recrystallization of amorphous silica in pure water and water solutions of some electrolytes.
Synthesis conditions of fine-crystalline quartz powder of preset dimensions and habit plane were detailed in experimental studies via recrystallization of amorphous silica. Crystal shaping was studied in the range of temperatures from 400 to 900oC and pressures from 0.4 to 5 knar under isothermal conditions. The runs of duration from 5 to 15 days were conducted in autoclaves (T to 700oC, P to 1 kbar), hydrothermal (T to 800oC, P to 3 kbar) and gas (T to 900oC , P to 5 kbar) high pressure apparatus. The operating condition was achieved at a heating rate 100-120oC/h (autoclaves) and in 30 min in gas HPA. After the runs the autoclaves were quenched in cold running water or in a compressed air jet (in order to prevent autoclave break-down). The mineralizers were H2O, NaOH (conc. from 0.006 to 5.8 %) and NaCl (conc. from 0.05 to 2 %).
The studies showed a significant effect of mineralizers and their concentrations on the degree of recrystallization of amorphous silica, on the occurrence of particular polymorphic silica modifications (cristobalite, tridymite, quartz, and paramorphs 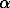 -quartz on
-quartz on  -quartz), on crystal dimensions and their habit planes.
-quartz), on crystal dimensions and their habit planes.
In pure water and in low-concentrated NaCl solutions (0.006-0.058 %) in the temperature range 400-900oC at a pressure 0.4 kbar cristobalite formed; at pressure increase to 1.5 kbar and temperatures above 700oC there appeared well-faceted prismatic crystals of quartz. With the pressure increase to 3 kbar such crystals formed in pure water but already at T=500oC.
#The work has been sponsored by the RFBR (Grant N 97-05-64805).
##The work has been sponsored by the RFBR
82
It was shown that in sodium chloride solutions an increase in the NaCl concentration from 0.006 to 5.8% in the temperature range 500-700oC leads to replacements of crystobalite by quartz of preferentially obelisk shape with, normally, developed faces of acutest rhombohedra, ordinary rhombohedra, and hexagonal prism.
No unambiguous dependence of crystal shape variation with changing temperature was observed in amorphous SiO2 recrystallization studies in NaOH solutions. It was, however, observed that at 500oC in the concentration range 0.05-2%, in all the runs, there formed quartz prismatic crystals of two kinds: small (0.01-0.03 mm) and large (0.1-0.3 mm). As the temperature elevated to 700oC obelisk-shaped-crystals measuring from 0.005 to 0.2 mm formed in less concentrated NaOH solutions ( 0.05-0.2%), and in 0.4-2% NaOH solutions there formed to a greater extent, short prismatic and to a lesser extent prismatic crystals.
As the concentration of NaOH solutions increased (from 0.05 to 2 %), the crystal sizes decreased drastically, their habit plane changing from obelisk-shape (combination of basic and acutest rhombohedra and hexagonal prism) to their mixture with isometric and then isometric with prismatic crystals.
So, the experiments suggest that the composition, density, and supersaturation of solutions are of no smaller effect on the change of crystal habit plane and the formation of new shaping faces than temperature and pressure are. This, most probably, is responsible for the frequently found in natural quartz discrepancy between the real crystal morphology and common ideas, of its relationship with only thermobaric parameters of crystallization process.
Besides, the studies have established optimal conditions for formation in the above solutions of monodisperse completely decrystallized quartz powder which is a promising raw material for synthesis of special purity quartz glass.
#Emel'chenko A.G., Makhina I.B., Balitsky V.S., Zharikov E.V. Experimental study of low temperature pink quartz growth conditions.
We have completed our experimental studies of specific growth features of rarest species of natural phosphorus-bearing pink quartz. The studies suggest that growth conditions of such crystals are quite peculiar. Primarily, this peculiarity is that their growth is realized only from acidic and near-neutral solutions on basic seeds, phosphate and fluorite ions being simultaneously present in the solutions. The habit plane and morphology of crystals growth with phosphate admixtures do not, practically, differ from those of crystals synthesized from NH4F solutions. But the occurrence of phosphorus ions affects appreciably the basal surface of crystals and, consequently, their inner structure: rough regeneration relief at phosphorus concentration in a solution in excess of 3-4 g/l transforms to vicinal one. Phosphate ions, like in natural low-temperature pink quartz [1], directly participate in the formation of centers-firerunners of pink coloration (phosphorus content in the structure amounts to 300 ppm), and fluoride ions as well as acidic and near-neutral character of solutions impede the formation in crystals of Al-alkali centres of smoked coloration. Pink coloration shows up under ionizing radiation (saturation fluence is about 5 Mrad). The temperatures of crystals growth and of subsequent thermal actions on them do not have to exceed, at least, 350oC to prevent pink coloration centers from irreversible collapse, and the total ionising radiation fluence does not have, for the same reason, to exceed 10 Mrad. An important factor in the formation of phosphorus centers of pink coloration is, also, crystal growth rate. It does not have to exceed the values at which trapping of structural phosphorus impurity on a non-structural one is inversed (of the order of 0.2-0.3 mm/d) [2,3,4].
The formation in quartz crystals of phosphorus centers of pink coloration and their long-time existence are possible only with the observance of the above mentioned conditions.
Of all geologic objects, such conditions are realized only in some pegmatites of phosphate-fluoride type. This, possibly, accounts for rareness of phosphorus -bearing pink quartz in nature.
References:
Nekrasov A.N. Magnetization of single-phase spatially inhomogeneous ferrimagnetic grains and self-reversal of residual magnetization of rocks.
An equation has been derived for an equilibrium of one-domain state (ODS) and non -one-domain state (NODS). The equation suggests that the transition of ODS to NODS and back is possible only in a single-phase spatially inhomogeneous ferrimagnetic grain (SPSIFG). The ODS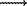 NODS transition is a first-order magnetic phase transition characterised by a decrease/increase of the SPSIFG magnetic moment (magnetization "anomalous peak"), release/uptake of thermal energy, and increase/decrease of the magnetic susceptibility ("false" Hopkinson peak). The ODS
NODS transition is a first-order magnetic phase transition characterised by a decrease/increase of the SPSIFG magnetic moment (magnetization "anomalous peak"), release/uptake of thermal energy, and increase/decrease of the magnetic susceptibility ("false" Hopkinson peak). The ODS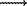 NODS transition temperature (Tcrit.) decreases with increasing external magnetic field (Hcrit.) and increases with increasing SPSIFG inhomogeneity degree. The values of Tcrit. and Hcrit. grow with the volume of a homogeneous ferrimagnetic grain.
NODS transition temperature (Tcrit.) decreases with increasing external magnetic field (Hcrit.) and increases with increasing SPSIFG inhomogeneity degree. The values of Tcrit. and Hcrit. grow with the volume of a homogeneous ferrimagnetic grain.
#The work has been sponsored by the RFBR (Grant N 97-05-64805).
83
The derived ODS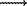 NODS equilibrium equation suggests that remagnetization nuclei (subdomains ) possess a higher mean Curie temperature <Tc> that <Tc> of the rest of SPSIFG. So, in NODS the domains whose magnetic moments are opposed to a magnetic grain in ODS have higher <Tc> than the rest of the domains. The subdomain size decreases as Hcrit increases, and increases with increasing Tcrit .
NODS equilibrium equation suggests that remagnetization nuclei (subdomains ) possess a higher mean Curie temperature <Tc> that <Tc> of the rest of SPSIFG. So, in NODS the domains whose magnetic moments are opposed to a magnetic grain in ODS have higher <Tc> than the rest of the domains. The subdomain size decreases as Hcrit increases, and increases with increasing Tcrit .
The results of numerical modelling of the equilibrium magnetic moments distribution inside SPSIFG in NODS have shown that the occurrence of considerable energy gradients of domain boundaries (primarily associated with the Curie temperature gradient Ñ Tc) leads to their displacement in the region of lower Tc values and, consequently, to a magnification of magnetic moments of the reversely magnetized domains. At Ñ Tc, appreciable in the absolute value, the complete self-reversal of residual magnetization of rocks can take place (SRMR).
The features of this SRMR mechanism are, first, the occurrence of the ODS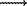 NODS transition in weak magnetic fields at a particular Tcrit.
NODS transition in weak magnetic fields at a particular Tcrit.
Second, as H increases Tcrit. decreases. Third, partial thermoresidual magnetization thermodemagnetization curves 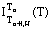 formed at Tn+1>Tn>Tcrit (H) demonstrate a characteristic "anomalous peak of thermoresidual magnetization", and at Tcrit.(H)>Tn+1>Tn thermodemagnetization curves
formed at Tn+1>Tn>Tcrit (H) demonstrate a characteristic "anomalous peak of thermoresidual magnetization", and at Tcrit.(H)>Tn+1>Tn thermodemagnetization curves 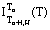 have no such peak but have clear "tails".
have no such peak but have clear "tails".
The obtained theoretical results were checked experimentally in studies of the dependence of Tcrit on an external magnetic field (H) for synthetic (synthesized by Konilov A.N.) and natural hemoilmenite (picroilmenite) specimens having chemical compositions 0.1<Xhem<1. The same studies were conducted for natural and synthetic specimens of titanomagnetite (0.2<Xmag<1). The temperature Tcrit of the investigated synthetic and natural specimens of Fe-Ti-oxides of hemoilmenite decreases with increasing external magnetic field and increases with an increase of the values characterizing a mean fraction of the specimens <Xhem> or <Xmag>. An increase in the specimens inhomogeneity degree (standard deviation of the chemical composition distribution 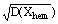 leads to an increase in Tcrit. For synthetic hemoilmenite specimens at H=0 Oe (0 A/m) the correlation factor between the parameter Tcrit-<Tc> (Tc is the Curie temperature corresponding to <Xhem> and
leads to an increase in Tcrit. For synthetic hemoilmenite specimens at H=0 Oe (0 A/m) the correlation factor between the parameter Tcrit-<Tc> (Tc is the Curie temperature corresponding to <Xhem> and 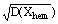 at H 700 Oe (55.7 kA/m ) R[Tcrit-<Tc>,<Xhem>]=0.88, at H=1.2 kOe (95.5 kA/m) R[Tcrit-<Tc>, <Xhem>]=0.90. In studies of the dependence of magnetization of a hemoilmenite specimen, having a mean chemical composition <Xhem>=0.72 and a standard deviation of the chemical composition distribution
at H 700 Oe (55.7 kA/m ) R[Tcrit-<Tc>,<Xhem>]=0.88, at H=1.2 kOe (95.5 kA/m) R[Tcrit-<Tc>, <Xhem>]=0.90. In studies of the dependence of magnetization of a hemoilmenite specimen, having a mean chemical composition <Xhem>=0.72 and a standard deviation of the chemical composition distribution 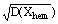 =0.12, on the temperature in the presence of an external magnetic field H=1.2 kOe (95.5 kA/m) at Tcrit=332oC (605K) an anomalous 40% increase of the specimen's magnetization was observed. The increase is accompanied by a drastic retardation of the specimen heating which corresponds to thermal effect ΔQ=0.8 - 1.4 J/g. At cyclic thermodemagnetization of the residual saturation magnetization Irs of some synthetic hemoilmenite specimens having mean chemical compositions in the range 0.40<Xhem<0.46 the phenomenon of self-reversal of residual magnetization was observed. The Io/Is value that characterises this effect decreases from 0.002 to -0.018 as the inhomogeneity degree
=0.12, on the temperature in the presence of an external magnetic field H=1.2 kOe (95.5 kA/m) at Tcrit=332oC (605K) an anomalous 40% increase of the specimen's magnetization was observed. The increase is accompanied by a drastic retardation of the specimen heating which corresponds to thermal effect ΔQ=0.8 - 1.4 J/g. At cyclic thermodemagnetization of the residual saturation magnetization Irs of some synthetic hemoilmenite specimens having mean chemical compositions in the range 0.40<Xhem<0.46 the phenomenon of self-reversal of residual magnetization was observed. The Io/Is value that characterises this effect decreases from 0.002 to -0.018 as the inhomogeneity degree 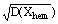 grows from 0.016 to 0.078. For these samples the correlation factor between
grows from 0.016 to 0.078. For these samples the correlation factor between 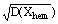 and Io/Is is (-0.84). At the same time the correlation factor between mean chemical compositions of these specimens <Xhem> and Io/Is is 0.03.
and Io/Is is (-0.84). At the same time the correlation factor between mean chemical compositions of these specimens <Xhem> and Io/Is is 0.03.
As the complete SPSIFG magnetic moment depends on its inhomogeneity degree, the changes in the latter caused by physicochemical processes over the geological time-period may lead, even in the presence of constant weak external magnetic field, to changes in magnitude and sign of natural residual magnetization of rocks, herewith the reversal of sign can occur repeatedly. Apparently, precisely the considered SRMR mechanism with a mobile domain wall can be responsible for a large number of the known cases of SRMR in natural rock specimens.
Romanenko I.M. Technique of X-ray microanalysis (EPMA) of Th, U, and Pb in monacites for the age determination
A PCMA technique for determination of low concentration of minor elements Th, U, and Pb in monacites and zircons has been developed. Concentrations of Th, U, and Pb help to identify the age of the analyzed mineral in different points. The test of age determination validity shows a good agreement with the traditional mass-spectrometry methods on the same samples. The errors of dating by the EPMA method approximately two times exceed the errors of traditional methods, however, it is quite enough to solve many geological problems. (Calculation of age from EPMA data was performed by A.Konilov).
The rate of determinations was enhanced by an order of magnitude using a complex technique. The microanalysis of major matrix components was performed by the energy-dispersion spectrometer, EDS with Si(Li)detector (Link AN10000, UK ). Th, U, and Pb were measured in the same points using the wave spectrometers because the limit of detection of elements in this case is an order of magnitude better than that of EDS.
#Chichagov A.V., Varlamov D.A., Dilanyan R.A., Narymbetov B.Zh., Dokina T.N., Drozhzhina N.A., Samokhvalova O.L., Ushakovskaya T.V. Mincryst - universal
information-calculating system on crystal structure data for mineralsMincryst is an original correlative combination of the Crystal Structure Database, Calculated Powder X-ray Diffraction Standard Subbase, and Applied Program Package.
#The work has been sponsored by the RFBR (Grants N 96-07-89162, 96-07-89323).
84
The Information Fund contains 4.6 thousand records in the Crystal Structure Database. The full version of the Mincryst is intended for the use on the individual (local) PC. The Crystal Structure Database - the main Mincryst component - is located on the IEM RAS Server and is accessible at the Internet site (http://database.iem.ac.ru/mincryst).
The main lines on the Mincryst development in the year 1998 were as follows:
1. Information Fund Supplement. Presently Mincryst includes 4.6 thousands records (2,300 unique minerals and 2,300 varieties in composition, structure, and PT synthesis conditions). The work concerned with search and correction of Mincryst Database errors has been continued, the records in the both Mincryst versions have been correlated with account of the fact that the record number is unique and is not subject to alteration.
2. Local version. The Mincryst Local Database has been transformed to Paradox 5.0 format and important to DELPHI 2.0 Management system (visual compiling environment to work out Windows supplement). The Mincryst Main Database (MAINDB) and the User Database (USERDB) are managed in terms of the universal System. The work with the both bases is identical but record editing is allowed only to the developer.
DB-record in the form of Information Card has been divided into four parts:
- Main (Mineral name, Specification, Crystal Chemical Formula, Space Group, Syngony, Unit Cell Parameters, Number of Formula Unit, X-ray Wave Length for CPDS, Angle Interval for Calculation of X-ray Powder Diffraction Pattern);
- Atomic positions (basic atomic positions coordinates);
- BDP (Calculated X-ray Powder Diffraction Standards);
- References (S-reference to Crystal Structure Determination, L-to Unit Cell Parameters Refinement, R-Reserve).
The Management System maintains the following regimes via text menu and/or special buttons:
a) MainDB regime provides access to Mincryst Main Database;
b) UserDB regime provides access to User Database, its supplement and editing included;
c) Characteristic List regime enables the User's look up of the contents of the both Bases according to the list containing the card number, mineral name, specification, and crystal chemical formula;
d) Parallel Browser regime enables the User's concurrent look up of the characteristic list and related mineral cards in scroll mode (fig.1);
e) Mineral Card regime enables the display of all the four mineral records (displaying to as many as 10 cards simultaneously is possible);
f) Import regime enables the Developer to import one new card or group of cards to the Main Database, and the User to import one card to the User Database;
g) Export regime enables the USER to export the Mineral Card (in the form of BDM- and BDP-ASC files) to the corresponding Subdirectories on a hard disc;
h) MinCalc regime. With built-in TRANS- and XRAYPOL-programs. INFO CARD, TRANS.DAT, BDP, BDI, HKL, and BDQ in the form of ASCII-files can be calculated and exported (fig.2);
i) RENTPOL regime. The formation and export of RENTPOL-file in special subdirectory can be performed and subsequently used for Quantitative X-Ray Phase Analysis [1,2];
j) Search regime enables the search for Mineral Cards in particular fields: mineral name, specification, crystal chemical formula (fig.3), space group, syngony, references, and card number;
k) Editor regime enables the Developer to edit the Mineral Cards in the Main Database.
The Applied DOS-Package main programs has been adapted for work under Windows. WinCrystpic-program displays the crystal structure on the monitor screen. WinMixipol-program calculates and displays full X-ray Powder Diffraction Profile of mineral or Mineral mixture in polycrystal-form.
3. Mincryst WWW-version.
Mincryst Database converted from the PARADOX 5.0 format into the SQL-format is located on the IEM RAS Server (http: //database.iem.ac.ru). The Mincryst Database WWW-interface has been created on the basis of the following Hardware and Software [3]: Work Station DEC Alpha LX-533, OS Digital Unix, http-server APACHE, Script Language PHP and JavaScript, DB-server mySQL.
The interface provides the global access to Mincryst Database with any graphical WWW-browser supporting Java 1.1.x. Search and selection of records are performed by the USER but records edition and input are performed by the DEVELOPER only.
Database ideology and regimes in the WWW-version are, in principle, analogous to those in the Local version. There are, however, marked differences. Primarily, a powerful search regime is available. In particular, a complex search for the chemical composition, reflected in the crystal chemical formula, and interplanar distances, contained in the polycrystal-standard Subbase, is, factually, adequate to the automatic qualitative X-ray phase analysis (fig.4). The Java-applets enable the transmission the crystal structure model image and the X-ray diffraction powder Line-Diagram image from the Server to the Client PC at a rate of the text information translation. The WWW-version of the Mineral Card has been supplemented by two new parts: Structure and X-ray Line Diagram (fig.5). Accordingly, two new regimes are operative.
The regime Structure enables the display of the crystal structure model (fig.6a) in the automatic mode for every mineral in the Database and subsequent manipulation with this image in the interactive mode (fig.6b). The Line Diagram regime enables the same operations to be performed for the model of the X-ray powder diffraction patterns (fig.7).
Conclusion. Mincryst is a powerful tool for an X-ray crystallographer to be used in X-ray diffraction powder analysis, qualitative and quantitative, and crystal chemical analyses. The work is under way for updating the Mincryst Database and Program Package.
More detailed information about Mincryst can be obtained at the address: Chichagov A.V. or Varlamov D.A., Institute of Experimental Mineralogy,142432 Chernogolovka, Moscow distr., Russia. E-mail: avch@iem.ac.ru (Chichagov), dima@iem.ac.ru (Varlamov). WebSite of Mincryst: http://database.ac.ru/ mincryst
Mincryst has been registrated in Database State Register (WWW-version N 0229805169, Local version N 0229805170) Scientific Technical Center "Informregister", State Committee of Russian Federation on Communication and Informatization.
85
Fig. 1. MinCryst local version. Parallel Browser Regime. Characteristic List (4514 records) and Information Cards are overlooked in Scroll Mode.
Fig.2. MinCryst local version. MinCalc Regime. INFO CARD, TRANS.DAT, BDP, BDI, HKL and BDQ files (in form of ASCII-files) with are calculated with built-in TRANS- and XRAYPOL-programs.
Fig.3. MinCryst local version. Search Regime. Searching of Information Card is carry out on Set of Chemical Element in Crystal Chemical Formula.
86
Fig. 4. MinCryst WWW-version. Search Regime. Searching of Information Card is carry out on Set of Crystal Chemical Characteristics.
Fig. 5. MinCryst WWW-version. Information Card Regime. Information Card is displayed.
Fig. 6. MinCryst WWW-version. Crystal Structure Image Regime. Image of Crystal Structure Model is performed with Java-applet (a). Interactive rotation of Crystal Structure Model is performed with Java-applet (b).
87
Fig. 7. MinCryst WWW-version. Line-Diagram Image Regime. Image of X-Ray Diffraction Powder Line-Diagram is performed with Java-applet.
References:
#Kalinichev A.G. Molecular clusters in supercritical water: size, topology, and hydrogen bonds.
A method of computer MD (molecular dynamics) simulation is used to study the size and topology distribution of molecular hydrogen-bonded clusters in water under supercritical conditions (at densities 0.17-1.28 g/cm3 and pressures 25-3000 MPa). The algorithm and the computer program were developed to carry out the calculations [1]. The quantitative analysis of the MD simulation results, performed for the first time with the use of the algorithm shows that:
1. Even at relatively low densities (less than 0.2 g/cm3) one can observe in the supercritical water a considerable amount of clusters of a rather large size (up to 10 molecules).
2. In low-density vapor-like state there are small isolated clusters. The compression of the fluid results in appearance of high-density liquid-like fluid characterized by the infinite cluster. A transition has been observed resembling the condensation phenomenon .
3. An analysis of abundance of clusters of various structural types shows that linear (chain) structures are dominant in the supercritical water. No cyclic molecular structures were found among five molecular clusters, and the fraction of pentamers in the form of hollow tetrahedra is insignificant.
4. A relative abundance of various topologic types of low-molecular clusters is practically density-independent.
The quantitative analysis of the lifetime of hydrogen bonds suggests that in supercritical water it is by one order of magnitude less than the similar parameter of liquid water under normal conditions and makes up 0.2-0.5·10-12 s.
The Monte-Carlo simulation technique is used to study the structures and specific features of hydrogen bonding in water under high hydrostatic pressures (up to 10 kbar). The simulation shows that the network of hydrogen bonds does not change appreciably, i.e. a mean number of bonds per molecule nHB = 3.2 throughout the entire pressure range. A density increase under these conditions is achieved, mainly, at the expense of more effective packing of the near-neighbour non-bounded molecules.
Reference:
##Novikov G.V., Sipavina L.V., Sokolov Ju. A. Comparative crystal-chemistry of mantle silicates and their structural analogs.
The goal of the study was the detailed investigations of structure and chemical bonding features in Ge-analogs of mantle related silicates. Authors intended to follow the changes in properties of germanates, attended (induced by) the composition or the temperature variation, both at the phase stability fields and at the phase transitions. Three types of structure were distinguished and characterized for the Fe-Mg and Fe-Ca germanates with pyroxene structure using the x-ray and neutron diffraction methods. For the FeGeO3 , which is the structural analog of the nonretainable high-pressure ferrosilite structure, the neutron diffraction data analysis was completed in the temperature interval 1.7-300 K, and two distinct magnetic structures were determined. At 4.2-300K the 57Fe gamma-resonance spectra were investigated for the Fe-Mg and Ca-Fe chain germanates, ferrosilite and hedenbergite, the hyperfine parameters of 57Fe nuclei at Ì1 and at Ì2 crystallographic positions were determined.
1. According to results of comparative analysis of topological X-trends Unit cell parameter Volume (Õ = Ni, Mg, Co, Fe, Mn, Ca) for germanates and relative pyroxenes with orthorhombic (s.g. Pbca) and with two distinct monoclinic structural modifications (both with the s.g. C2/c) the structures and the features of their deformation (X-trends) at isomorphous substitution are very close for germanates and corresponding silicates. In particular, as in silicates, two different monoclinic chain structures ('C2/c-I' è 'C2/c-II') with the same space group were determined in the Fe-Ca germanates (Fig.1).
#The work has been sponsored by the RFBR Grant N 97-03-32587 and INTAS grant N 96-1989.
##The study was supported by RFBR, grant N 98-05-64588.
88
Mg è Fe
Fig. 1. Õ-trends c - V and  - V for the monoclinic (s.g. C2/c) Mg-Fe germanates (squares) and silicates (circles). The modifications 'Ñ2/c-I' (dotted line) and 'Ñ2/c-II' (line) yield trends with the definitely distinctive character.
- V for the monoclinic (s.g. C2/c) Mg-Fe germanates (squares) and silicates (circles). The modifications 'Ñ2/c-I' (dotted line) and 'Ñ2/c-II' (line) yield trends with the definitely distinctive character.
The very close features of the X(Mg => Fe) trends for silicates and germanates (along with other data) permit one to propose that as an indirect prove of close physical properties, in particular, to propose close properties of the strain tensors for the germanates and relative silicates. This, in turn, could support the idea to use the structural analogs in tasks of mantle mineralogy.
2. The second goal of the study was to investigate the features of the electron structure of the Fe ions in every of 4 known structural modifications of chain structures, and to determine the possible role of bonds metal oxygen at phase transitions in pyroxene structures. It was shown, that in all these 4 pyroxene structures, in which Fe ions are the host ions (including nonretainable high-P clinoferrosilite structure) the mean length of the Fe-O bond in the M1 octahedra does not exceed the value of 1.3A. In any case, the phase transitions Pbca à C2/c-II and P21/c à C2/c-I at high pressure take place at reaching just this value of the bond length in the 'mother'phase. That is why the local field parameters at Fe atoms in the M1 positions were studied in more detailed.
For three structural modifications of pyroxenes and their Ge-analogs the comparative analysis of Fe2+ electronic structure was performed. It was shown, that the mutual dependence of quadrupole splitting (Q.s.) and the valence electron density on 57Fe nuclei (I.s.) is specific enough to be used both at comparative analysis of the Fe 2+ electron shells and at the direct identification of the pyroxene structure modification itself.
Using the features of the Q.s. I.s. trends for silicates and germanates with pyroxene structure three types of electronic structures of Fe 2+ ions were distinguished: C type in the structure C2/c-II (hedenbergite and their Ge-analog, short dotted lines); 'B' type in the orthoferrosilite structure and in the high-pressure low-clinopyroxene P21/c structure, which can be saved by
89
quenching (dotted line); 'C' type in the high pressure monoclinic structure of ferrosilite (C2/c-I), which can not be saved by quenching, and in stable at normal conditions ferrogermanate with the same structure as well in the orthogermanate (line). Three specific types of bonding Fe2+ Î in M1 positions can be proposed for three types of Q.s. I.s. trends distinguished. Using the Q.s. I.s. trends it was shown, in particular, that in M1 positions of Ca-Fe germanates simultaneously exist two distinct electronic states of Fe2+ ions (M1 and M1a , Fig 2.)
In general, the role of chemical bonding just in this positions M1in pyroxenes is, apparently, very important. According to nuclear gamma-resonance data, at phase transitions Pbca à C2/c-II and P21/c à C2/c-I in pyroxenes and at phase transition C2/c-I à C2/c-II in germanates the type of Fe2+ electronic structure is changing just in the M1 positions, what is illustrated by T- trends Q.s. I.s. (Fig 2.)
Fig 2. Tree types (A lines; B dotted lines; C short dotted lines) of T trends Q.s. I.s. for silicates (circles) and germanates (squares) with pyroxene structure. Data at 80K 300K were used. For the high pressure structures of ferrosilite (C2/c-I small circles, P21/c rhombs) the in situ data (P=1.74 - 3.84 GPa, Ò=300Ê, McCammon and C. Tennant, 1996) were used. The in situ data on hedenbergite (Li Zhang and S.S. Hafner, 1992) are presented as well.
90