I. Magmatic systems and petrology of the silicate melts (Leader Academician V.A.Zharikov)
#Litvin Yu.A., Litvin V.Yu. Experimental high-pressure study of the interaction of alkali-carbonate and alkali-alumosilicate components of plumes with rock-forming minerals of the lithosphere in the Earth's mantle hot spots.
Phase equilibria in alkali carbonate - silicate systems Fo-K2CO3 and Fo-K2CO3-K2SiO3 which model interactions of chemically active alkali-fluid components of plumes with the basic rock-forming mineral of the lithosphere (mantle hot-spot situation) [2,3] have been experimentally studied at 40 kbar. It has been found that magnesite MgCO3 and the K2Mg(CO3)2 phase which originate three four-phase associations including forsterite-magnesite (0-55 mole% K2CO3), magnesite-K-Mg-carbonate (44-67 mole% K2CO3), K-Mg-carbonate (67-100 mole% K2CO3) form in a subsolidus. These processes of high-temperature mantle metasomatism can give rise to intensive processes of mantle carbonatization in the interface zones between chemically active mantle plumes and host mantle. Eutectic melting relations of solidus mineral associations (composition of the eutectic silicate melt corresponds, e.g. to SiO2 - 55.47, MgO-29.14, K2O-15.39 wt%) are found under forsterite-carbonate systems melting conditions. In runs with more complete melting one observes isolation of carbonate melts from silicate ones (liquid carbonate-silicate immiscibility). This gives grounds for a tentative conclusion about physicochemical conditions of formation of primary magmas of alkali carbonatites, basalts, and hybrid carbonate-silicate kimberlite rocks including diamond-bearing ones.
Phase equilibria in alkali alumosilicate-silicate enstatite-nepheline system [1,4,5] modeling boundary interactions in high-temperature halos of mantle hot spots and the nepheline-normative mantle have been studied. The system is a pseudobinary inner join of the basic ternary forsterite-nepheline-SiO2 system and demonstrates subsolidus interactions of two types: a) low-temperature one to 1500oC with the formation of forsterite and jadeite and their associations with enstatite and nepheline; b) high-temperature one at 1500-1600oC with the formation of the new compound Na2Mg2Si2O7 (NMS-phase), pyrope, and enstatite component- a constituent of a jadeite-based clinopyroxene solid solution. The solidus ratios of the system are governed by the peritectic reaction Fo+melt=Na2Mg2Si2O7 , the peritectic melt composition in the nonvariant point varies in microprobe determinations, the composition: Na2O -15.72 MgO - 11.92, Al2O3 - 16.03, SiO2 - 56.33 wt% is typical. The obtained results can serve as the base for models of alkali: magmas generation in the nepheline-normative mantle and hot spots and for differentiation of alkali mantle magmas (with loss of olivine). Also, the obtained results support the possibility of lithosphere garnetization processes as a result of Na-alkali reactions of mantle olivine and orthopyroxene.
References:
##Litvin Yu.A., Chudinovskikh L.T., Saparin G.V., Chukichev M.V., Shiryaev A.A. Experimental study of crystallization conditions, mechanism, and physical properties of diamond synthesized in alkali-carbonate systems under high pressures.
Crystallization of diamond was first accomplished in carbonate-carbon melts of the Na2Mg(CO3)2-K2Mg(CO3)2-C (graphite) system at 80-100 kbar [1,2]. Spontaneous crystallization gave rise to colorless transparent octahedral crystals of diamond measuring to 0.15 mm. Runs were also performed on diamond growth from carbonate-carbon melts of the system on cubo-octahedral single-crystalline diamond seeds (0.5-0.7 mm) synthesized in metal (Ni50Mn50 wt %) - graphite system. It has been found that diamond crystallization
#The work has been sponsored by the Federal programm "Integration" (project N 250), RFBR (project N 96-05-64986) and ISSEP grant (NS 99-564) for the Soros student V. Yu. Litvin.
##The work has been sponsored by the Federal Program "Integration" and the RFBR (project N 96-05-64786).
23
in carbonate-carbon systems under high pressures proceeds by a solution-melt mechanism (dissolution of graphite in a carbonate melt, diffusion-controlled carbon transfer, diamond crystallization from supersaturated carbon solutions - this mechanism is consistent with the data on carbon isotopy in starting substances and in run products, obtained in the V.I.Vernadsky Institute of Geochemistry and Analytical Chemistry RAS [6]). Here, spontaneous nucleation occurs in the kinetic region of labile solutions (RLS) and top-seeded growth in the kinetic region of metastable supersaturations (RMS). It has been found that the lower-pressure spontaneous crystallization boundary in the Na2Mg(CO3)2-C system is at 85 kbar for 1600oC [5] which is 10 kbar lower than in the K2Mg(CO3)2-C system. Morphology studies of diamond crystals synthesized in carbonate-carbon systems clearly demonstrate that deposition of nascent diamond layers occurs on octahedral faces which form being crystallomorphologically "even" [2,4,7]. Contrastingly, overgrowth of cubic faces is effected by crystallomorphologically "uneven" layers consisting of tightly intergrown octahedral microcrystals. This way for the crystallizing material to deposit on cubic faces is not characteristic of synthetic metal-carbon diamonds but is established for natural diamonds. Principally new results were obtained in CCL-SEM and CL-spectroscopy studies [4,6]. CCL patterns demonstrate either the absence of color cathodoluminescence characteristic for nitrogen-bearing synthetic metal-carbon and natural diamonds or the occurrence of red luminescence of very low intensity on octahedral faces and of strong one on uneven cubic faces. These features of color cathodoluminescence are characteristic of solely carbonate-carbon synthesized diamonds. Cathodoluminescence spectra for carbonate-carbon diamonds exhibit a 360-650 nmk band with a low-intensity blue line, a 440-480 nmk band with a maximum migrating within the band, and an intensive evidence derived from studies of physical properties of carbonate-carbon diamonds. We can conclude that their properties are close to most pure "nitrogen-free" natural diamonds of the II type.
References:
#Chudinovskikh L.T., Matveev Yu.A., Litvin Yu.A., Perchuk L.L., Yapaskurt V.O. Experimental investigations related to the problem of diamond-bearing calc-silicate rocks of the Kokchetav metamorphic complex.
The following data formed the base of our experimental studies: the presence of alkaline carbonate inclusions in diamonds from kimberlites [1], systematic presence of potassium clinopyroxenes (K-Cpx) in diamondiferrous rocks from kimberlites [2] and from the Kokchetav complex [3,4], and crystallization of diamond in alkali-reach carbonic systems with graphite [5, 6].
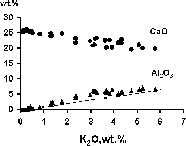
Fig.1. Correlations between K2O, CaO and Al2O3 for K-Cpx from runs at P=7 GPa and T=1110-1710oC.
The method of investigations and apparatus are described in [6]. The system K2CO3-CaMgSi2O6-Ca3Al2Si3O12 has been studied at a pressure (P) of 7 GPa and temperature within the range of 1110-1710oC. The run products are K-Cpx, grossular-pyrope garnet (Grt), dolomite (Dol), potassium-bearing silicate phases, i.e. K-Wadeite K2Si4O9 (K-Wad) and K(Ca, Mg)AlSi2O7 (K-sil) and quenched melt (Q) (Table 1).
#This work supported by the Russian Foundation for the Basic Research (grant N 98-05-64033). The authors are grateful to E. V. Guseva, N. N. Korotaeva (Moscow State University), A. P. Jones and A. Beard (University College London) for their help in this work.
24
Table 1. The results of experiments in the system CaMgSi2O6 Ca3Al2Si3O12 K2CO3 (for starting composition Di25Gross25(K2CO3)50) at 7 GPa and 1110 1710oC
_____________________________________________________
Run No T,oC Run duration, min Run products
________________________________________________________________
267 1710 20 K-Cpx +Di + Grt + K-Wad + Q
268 1600 40 K-Cpx +Di + Grt + K-Wad + Q
264 1500 40 K-Cpx +Di + Grt + K-Wad + Q
259 1400 50 K-Cpx +Di + Grt + K-Wad + K-sil + Q
258 1300 60 Di +K-Cpx + Grt + K-Wad + K-sil + Q
256 1200 60 Di +K-Cpx + Grt + K-Wad + K-sil + Q
265 1110 120 Di +K-Cpx + Grt + K-Wad + K-sil + Dol
Table 2. Electron-microprobe analysis data for clinopyroxenes with the highest potassium content (at 7 GPa)
T,oC |
1110 |
1200 |
1300 |
1400 |
1500 |
1600 |
1710 |
SiO2 |
54.47 |
54.78 |
54.51 |
55.42 |
53.72 |
54.85 |
55.43 |
Al2O3 |
6.12 |
6.35 |
5.96 |
6.51 |
7.14 |
6.51 |
6.02 |
MgO |
15.05 |
14.45 |
14.75 |
13.67 |
12.78 |
12.45 |
14.10 |
CaO |
20.75 |
20.27 |
21.10 |
20.15 |
19.94 |
19.74 |
19.39 |
K2O |
3.08 |
3.66 |
3.17 |
3.91 |
5.22 |
5.75 |
4.76 |
Total |
99.47 |
99.51 |
99.49 |
99.66 |
98.80 |
99.30 |
99.70 |
Cation ratio |
|||||||
Si |
1.96 |
1.97 |
1.96 |
1.99 |
1.97 |
1.99 |
2.00 |
Al |
0.26 |
0.27 |
0.25 |
0.28 |
0.31 |
0.28 |
0.26 |
Mg |
0.81 |
0.77 |
0.79 |
0.73 |
0.70 |
0.67 |
0.76 |
Ca |
0.80 |
0.78 |
0.81 |
0.78 |
0.78 |
0.77 |
0.75 |
K |
0.14 |
0.17 |
0.15 |
0.18 |
0.24 |
0.27 |
0.22 |
Total |
3.97 |
3.96 |
3.96 |
3.96 |
4.00 |
3.98 |
3.99 |
End members (mole percents) |
|||||||
K-Jd |
14.2 |
16.8 |
14.8 |
18.2 |
24.6 |
26.6 |
22 |
Ca-Tsch |
3.5 |
4.9 |
3.0 |
1.1 |
3.1 |
0.7 |
- |
Ca-Esk |
4.8 |
1.8 |
3.4 |
7.4 |
- |
- |
3.6 |
Di |
74.4 |
74 |
76.5 |
73.2 |
69.7 |
67.6 |
73.1 |
En |
3.1 |
4.5 |
1.3 |
0.1 |
- |
- |
1.3 |
Wol |
- |
- |
- |
- |
2.6 |
5.1 |
- |
The K-Cpx contains up to 5.7 wt. % K2O (Table 2) (compared with the data from [7, 8]). Both the Al <=> K positive correlation and the Ca <=> K negative correlation in the clynopyroxene solid solution reflect the CaMgSi2O6-KAlSi2O6 substitution [3, 7]. The Al/K ratio is higher than 1 that indicates Tschermak's substitution as well. The exess of silica was also observed in the K-Cpx (Table 2). Such Eskola's [9, 10] substitution, i.e. K-Cpx <=> Ca0.5 0.5AlSi2O6 presumably facilitates introducing K into M2 vacancies in the Cpx structure.
Our experimental results support an idea on the magmatic origin of Cpx-Grt diamond-bearing calc-silicate and coarse-grain silicate rocks from the Kokchetav metamorphic complex [3,4,].
References:
25
Khodorevskaya L.I. Fluid-magmatic interaction in rocks with basic composition.
The studies of fluid inclusions presented in numerous works showed that concentrations of solutions of salts in both magmatic and metamorphous fluids can significantly change from negligible to high values corresponding to brines [1-3]. In melting processes, the action of essentially aqueous fluids with a low amount of dissolved salts on processes of partial melting of the rock affects, evidently, the composition of melts and co-existing mineral associations. High concentrations of salts in the fluid leading to a decrease in the activity of water change substantially melting points of rocks [4, 5]. The influence of the salt loading of the fluid phase on the composition of melts and co-existing mineral phases is not virtually studied experimentally.
Our work is concerned with the experimental study of partial melting of the amphibolite [6] in the multicomponent fluid-silicate system, where the fluid composition was specified by solutions of HCl, NaCl, and CaCl2 with different concentrations. The experiments were carried out at 900oC and a pressure of 5-7 kbar on a high-gas-pressure setup with internal heating by the quenching procedure. The duration of experiments was 4 days. The procedure of experiments and methods for treatment of products are presented in [4].
The main regularities revealed in the work are the following:
- the presence of cations of salt in the initial fluid (NaCl, KCl, CaCl2) results in enrichment of the melt in the corresponding cation, and plagioclases are enriched considerably in the anortite component;
- the compositions of clinopyroxenes in all experiments, where salt was introduced into the initial fluid, are presented by diopside with the ferruginosity f = 27+2%. In the systems without water, with a water excess, and in the presence of HCl, clinopyroxene is augite with an Al content higher than 3 wt.%;
- the initial amphibole, ferro-pargasite mixture, remains its composition almost unchanged during melting of the amphibolite under dry conditions and in the water excess. As the concentration of NaCl increases, the composition of the amphibole changes as follows: ferropargasite mixture-ferro-pargasite--ecermanite--pargasite;
- the amount of amphibole decreases and that of pyroxene increases as the salt concentration increases at constant T-P parameters.
Microprobe analyses of all phases present in experiments make it possible to write equations of equilibrium reactions of the initial and newly formed (on the basis of mass balance) components recalculated to formula units. Since the melt participates in the equations, the authors recalculated its chemical composition and the composition of the initial amphibolite to the formula including 16 oxygen atoms.
Entry no |
fluid |
P, kbar |
equations of reactions |
C-14 |
- |
5 |
Amft init.. = 0.28 Cpx+0.37 Pl +0.39 Amf +0.13 Gl + OFe+0.001Opx |
V-8 |
H2O |
5 |
Amft init.. = 0.26 Cpx+0.41 Amf +0.26 Gl + OFe + 0.02 Fl |
G-2 |
1.5% NaCl |
5 |
Amft init.. = 0.51 Cpx+0.10Pl+0.35 Amf +0.22 Z + 0.01 Fl |
U-1 |
5.5% NaCl |
5 |
Amft init.. = 0.58 Cpx+0.01 Pl +0.28 Amf +0.34 Z + 0.12 Mt + 0.03Fl |
T-4 |
5.5% NaCl |
7 |
Amft init.. = 0.61 Cpx+0.01 Pl +0.24 Amf +0.36 Z + 0.15 Mt + 0.06Fl |
F-2 |
1.5% NaCl + CaCl2 |
7 |
Amft init.. = 0.91 Cpx+0.15Pl+0.13 Amf +0.34 Z + 0.03Fl+0.01Spl+OFe |
F-3 |
1%CaCl2 |
7 |
Amft init.. = 0.29 Pl +0.21Amf+0.80 Cpx+0.23Gl + 0.02 Fl+OFe |
F-1 |
10%CaCl2 |
7 |
Amft init.. = 0.23 Pl +1.18 Cpx +0.24Gl + 0.32Spl+0.08 Fl+OFe |
The following designations are used in the equations of reactions: Amft is the initial amphibolite; Opx and Cpx are ortho- and clinopyroxenes; Fl is fluid; Ne is nepheline; Mt is magnetite; Pl is plagioclase; Spl is spinel; OFe are iron oxides; Gl is the melt formed during partial melting of phases; and Z is the quenching phase that exists in experiments as droplets and can easily be determined by microprobe analysis.
The balance equations made it possible to determine the chemical composition of the lost substance by the equation: initial composition - composition of restite.
The data obtained can be used for the analysis of the granitization processes and partial melting of basic rocks in natural systems.
References :
26
Bezmen N.I., Kalinichev A.G., Zharikov V.A. Solidus of the system SiO2-NaAlSi3O8-H2O-H2 (Ptotal=2kbar)
The vapor-saturated solidus of SiO2-NaAlSi3O8-H2O-H2 system at 2 kbar over the range of gas phase composition from pure water to X(H2O)v=0.1 was studied (Fig. 1) in the internally heated gas-media pressure vessel.
Fig. 1 Solidus of Ab-Qz-H2O-H2 system. Melting curve of Qz-Ab eutectic under water fugacities egual in the runs (dot and dash line) was constructed from experimental data of this work (2 kb) and Schmidt et al. 1997 (1 kb).
In the experiments, various H2O/H2 compositions were controlled directly, rather than using solid phase buffers. The results show that the melting temperatures decrease in the X(H2O)v range from 1 to 0.7 compared to H2O-saturated albite under relevant water fugacity. At X(H2O)v=0.9 the solidus curve has a pronounced minimum with the temperature depression of 30oC as well as the melting curve of albite under pressure of H2O-H2 fluid (Bezmen et al., 1998). Further addition of H2 to the gaseous mixture leads to the increase of melting temperatures. In the region of WI-buffer albite melts at temperatures about 70oC higher than hydrous albite under the pure water fugacity equal the partial one in the H2O-H2 mixture. The melting point of 990oC in pure hydrogen has been calculated by an extrapolation of H2O-H2 data.
References:
Simakin A.G., Salova T.P. Experimental study of BSD evolution at a smooth degassing of a granite melt.
The scenario of the phase transformation dynamics is incomplete without studying phase size distribution. Size distributions predetermine important dynamic parameters such as mean phase transition rate, reaction surface [1]. Size distributions of crystals and bubbles in natural magmatic melts are used to estimate dynamic parameters of their formation [4,2]. Here we report original experimental data on the temporal evolution of granite melt degassing at the level of size distribution of the forming bubbles
Using a multicapsule gas bomb, we studied bubbling of a garnet melt at a constant-rate pressure release from 1000 down to 500 atm, T-750oC for approx. 1 day. The release smoothness was provided by a special mechanic reducer. Transparent polished sections were prepared from samples of each series consisting of 3-4 runs. Surface image of each section was obtained in a SEM at different magnifications and recorded on a computer. About 200 images were processed which made it possible to calculated 10 bubble size distributions (BSD).
It has been shown that the BSD form depends significantly on the number and size of air bubbles trapped in a glass at melting. In one series a small number of rather large bubbles were present in the initial glass. They, despite a decreased content of microbubbles, provided an effective removal of water from the melt. This group of large bubbles forms a "tail" in the distribution. Analogous tails are observable in crystal size distribution as well (microporphyry phenocrysts). In the other series (35) the final distribution is closer to exponential (Fig.1) typical for crystal size distribution and usually attributed to phase transition with gravitational removal of earlier large crystals and bubbles [4]. In the given case this interpretation is unacceptable.
Based on the obtained BSD and, also using primary data, we have estimated total sample porosity. In fig.2 are given the both estimates along with the theoretical values for water solubility in a granite melt at run parameters and, also, for water fluid density calculated by the model of the work [3] at different initial contents of water shown in the figures. It is seen that the bubbling degree in runs 35 and 31 was initially much lower than the equilibrium value.
The experiments conducted correspond to a slow ascent of granitic magma with viscosity 5.105 poise in a channel at a rate of approx. 0.02 m/s. Under the assumption that the degassing rate is proportional to viscosity, these conditions conform to a rapid and very rapid ascent of the basic magma with viscosity 5.103-5.102 poise at a rate of 2-20 m/s. Irrespective of the cause of the observable delay of the overall volume of the liberated fluid, our data suggest that at the ascent of the granitic magma and in the course of convective flows of water-bearing magma there can occur nonequilibrium effects in a magmatic chamber.
27
Fig.1. Summary bubble size distributions (calculated tree-dimensional BSD (series 35)).
Fig.2. Summary porosity of samples as a function of the run termination pressure. The upper continuous curve is the calculated values, symbols of the porosity estimates from the BSD count results and from relative pores cross-sections in series 31 and 35.
References:
#Salova T.P., Zavel'sky V.O. Mechanism of dissolution and forms of water occurrence in quartz glass.
PMR-spectroscopy and electron diffraction methods were used to study water-bearing quartz glass with 7 wt% H2O. It was supposed that at a short-time sample preparation water macroclusters (containing approximately 103 H2O molecules) form in the glasses which, subsequently, disappear at a longer run duration and there form OH-groups whose localization density is different. To check this supposition we synthesized water-bearing quartz glasses containing 7 wt% water: I-exposition time was 1 h; II-exposition time was 4 h.
The PMR spectrum of sample 1 demonstrates the predominance of a narrow singlet (=500 Hz wide). An analysis of the temperature behaviour of the spectrum suggests that the narrow singlet is formed by H2O molecules of water clusters, and the broad spectral component is formed by protons of OH-groups associated with silicon (fig1a, sample I, the spectrum was obtained at room temperature).
The relationship between the integral intensities of the macrocluster signal and the OH-groups signal is markedly dependent on the sample preparation conditions - the higher the temperature and the pressure at which the water fluid intrudes into the melt, the smaller is the amount of macrocluster water in the sample as compared with OH-groups. In sample II, obtained with 4 h exposition, no PMR-signal of macrocluster water is seen (fig.2, sample II).
a)
#The work has been sponsored by the RFBR (project N 98-05-64148).
28
b)
Fig.1. Sample I (tyeni=1h, T=1200oC, P=2kbar). a) PMR spectrum obtained at room temperature (1,2-narrow and broad components, respectively); b) experimental spectrum at T=130K (exp), its components (peaks 1,2,3) and calculated spectrum (dots).
Fig.2. Sample II (tyeni=4h, T=1300oC, P=6 kbar). a) experimental spectrum at T=450K (exp), its components (peaks 1,2), and calculated spectrum (dots); b) experimental spectrum at T=150K (exp), its components (peaks 1,2), and calculated spectrum (dots)
The temperature behaviour of narrow lines in spectra of water-bearing quartz glasses confirms their origin from water macroclusters. At cooling the sample this signal exists to T=240K practically without changes. At T=240K the narrow line disappears from the spectrum, that is, up to T=240K practically all macrocluster water is in liquid state. At further heating the narrow signal appears abruptly in the spectrum at T=275K. Noteworthy is a sharp temperatural hysteresis of the aggregate state of water in a macrocluster due, possibly, to the fact that practically all its molecules are acted upon by a field of surface at the water-glass interface. At a temperature much lower than the freesing point of macrocluster water (fig.1b, the spectrum was obtained at =130K ) the PMR signal of sample I consists of three components of different width and intensity. In this spectrum the broadest line formed, possibly, by frozen macrocluster water is predominant. The other two signals belong to protons of two differently localized OH-groups.
The spectra of sample II contain no signal of macrocluster water throughout the entire temperature range investigated. Irrespective of the temperature the spectra of this sample are two-componental. The both components are formed by OH-groups localized with different density. The temperatural spectrum alterations are only that the widths of its both components grow as the sample temperature decreases.So, the kinetics of the water diffusion transport into a quartz melt and the temperatural behaviour of narrow lines in PMR-spectra of water-bearing quartz glass suggest that these signals are formed by water molecules joined to clusters.
29
#Ishbulatov R.A., Suk N.I., Krigman L.D. Melting phase relation in the join Ol70-Cpx70-Pl70 at 0.2 kbar.
The results of moderate gas pressure (0.2 kbar) runs are comparable with the data obtained at 1 atm. The difference in the melting temperature of minerals is within the measurement accuracy since maximum dT/dP values of the melting lines do not exceed 15oC/kbar. At the same time even moderate pressures allow one to run the experiments in sealed capsules which is particularly important for alkali-containing systems.
The runs were conducted in a piston-cylinder-gas apparatus at P=0.2+0.05 kbar. In it the ordinary piston-cylinder cell with a graphite heater is used as a furnace, and the gas pressure (Ar) is produced by an independent compression source. The temperature was measured with a Pt70Rh30-Pt94Rh6 thermocouple with an accuracy +5oC and controlled automatically.
The starting materials were mechanical mixtures of preliminarily synthesized Cpx (70% mol. Di, 30% mol. Ged), Ol (70% Fo, 30% Fa), and Pl (70% An, 30% Ab). A mixture (~30 mg) was loaded in cylindric Pt capsules (dia 3 mm, h 5 mm) lined with tungsten foil (0.03 mm). The run duration was 3-12 days. The experimental results are illustrated in the figure in comparison with the Fo-Di-An diagram at 1 atm. The substitution of Fe for some part of Mg in Ol and Cpx and NaSi for Ca Al in Pl leads to broadening of the liquidus crystallization field of Cpx and drastic reduction of the Sp+L field. Accordingly, piercing Cpx+Pl+Ol+L (1195oC) and Pl+Ol+Sp+L (1230oC) points move forwards Ol-Pl side. The line of common Ol and Pl crystallization which connects these points is characterized by a very flat slope: 35oC at a change in the Cpx content from 5 to 48 wt% (in the system Fo-An-Di-45oC, the Cpx contents changes from 22 to 52 wt%).
Such temperatural relief of the Ol+Pl+L line explains the preferential formation of olivine and plagioclase phenocrysts at evolution of basalt melts (MORB) at small depths and a relative rareness of occurrence of clinopyroxene phenocrysts.
The extrapolation of the Cpx+Ol+L line to the edge join Cpx-Ol indicates a significant broadening of the Cpx liquidus crystallization field in this join as compared with nonferruginous system. This is associated with the fact that the Fe-for-Mg substitution decreases the melting temperature of Ol to a greater extent, compared to Cpx (the difference of the melting temperatures between Mg and Fe end members for Cpx is ~180oC, for Ol - 685oC ).
##Ishbulatov R.A., Krigman L.D., Nielsen T.F.D., and Veksler I.V. Melting relation on the joins Di-Phl and Di-Ks.H2O at 0.2 GPa.
Petrogenesis of potassium-rich rocks is modelled by phase equilibria of the system kalsilite-forsterire-larnite-quartz-H2O incorporating phlogopite, wollastonite, akkermanite, and leucite. The crucial part is played by equilibria with the participation of phlogopite. The crystal differentiation of silica-undersaturated melts with phlogopite g can give rise to larnite-normative liquids corresponding to melilitites.
Phase equilibria on the joins Di-Phl and Di-Ks.H2O were studied at 0.2 GPa and 900-1450oC. The runs were performed in a piston-cylinder-gas (Ar) apparatus. The run duration was 13-73 h. The run products were examined by optical, X-ray, and microprobe methods.
The starting materials were:
The latter two starting mixtures were produced by intermixing of Al(OH)3 and KMg3Al0.33Si3O10 and KAl0.33SiO3 glasses, accordingly, synthesized by melting of SiO2, MgO, K2CO3, and Al(OH)3 mixtures.
The run results are illustrated in fig.1.
The subliquidus topology of the Phl-Di diagram is dictated considerably by incongruent Phl melting behaviour by the reaction Phl=Fo+L with the melt enrichment in a leucite component.
The Di-Ks.H2O diagram structure is much more complicated which is related with the reactions in the subsolidus:
3Di+2Ks.H2O =Phl +Lc+3Wol+H2O (1)
6Di+5Ks.H2O =Phl +4Lc+3Ak+ 4H2O (2)
#The work has been sponsored by the RFBR (project N 97-05-64390).
##The work has been performed under INTAS-RFBR (95-953 projects and RFBR N 97-05-64390 project).
30
Accordingly, three subsolidus associations: Di+Phl+Lc+Wol, Phl+Ak+Wol+Lc, Phl+Ks+Ak+Lc are fixed in the join Di-Ks.H2O.
It is worth mentioning that no forsterite is found in the subliquidus region. The diopside-melt reactions are characteristic for compositions enriched in kalsilite component.
The results obtained enable one to construct petrogenetic models of evolution of potassium-rich melts under crustal conditions.
Fig.1. Preliminary T-x diagram of Diopside-Phlogopite join.
Fig.2. T-x diagram of Di-Ks*H2O join.
31