Radioactive wastes
#Suvorova V.A. and Kotel'nikov A.R. Synthesis of ceramic phosphate-containing matrices for immobilization of radionuclides of rare-earth elements (La, Ce).
key words [radionuclides of Ce and La immobilization ceramic matrix mountain rocks burial]
The purpose of the work was to study the possibility of binding radionuclides of rare-earth elements, lanthanum and cerium contained in waste of nuclear fuel, to ceramic materials by their synthesis from phosphates of imitators of the corresponding elements and easily accessible starting raw materials, natural mountain rocks.
The synthesis makes it possible to obtain a cheap final product corresponding to the principles of (1) multi-barrier character of protective compositions and (2) phase and chemical correspondence in the matrix-host rock system [1]. The multi-barrier protective compositions obtained consist of monazites and alumosilicates, each of which is a barrier for radionuclide loss, because it binds them chemically or mechanically; host rocks with which the ceramics synthesized is in the phase and chemical correspondence (equilibrium) is the third barrier.
For mass burial of highly radioactive waste (HRW), the problem of preparation of the material without high material and energy expenditures is still urgent. Ceramics obtained by caking of grains of minerals of finely grind rocks and calcinated radioactive waste can serve as this material. It was shown in [2] that rocks are appropriate for binding Na, Ca, Cs, and Sr from HRW. Based on the requirement of easy incorporation of lanthanides and REE in the structure of minerals, we chose for experiments the rocks containing alkali pyroxenes and silicates of REE, granites and tuffs.
Starting material: natural granites (Le2 and Le7), tuff (211A), and cerium and lanthanum orthophosphates synthesized by the special procedure [3].
Experimental. Silicate components and phosphates were grind in an agate mortar for 1.5 h until the uniform composition was achieved. Pellets with a mass of 1.5 g, height 5-6 mm, and diameter 8 mm were prepared from the mixtures at room temperature by the cool molding method at a pressure of about 100 kg/cm2. The pellets obtained were caked in platinum crucibles for 3 days at 1180oC in a KO-14 electroheating furnace (runs Fm1-Fm5), and runs Fm7-Fm8 were performed at 1040oC.
Analytical procedures. The complex thermal analysis of samples of the starting silicates was performed on a Q-1500D derivatograph, which decreased the ceramization temperature to 1040oC (the temperature of the phase transition).
All samples (both of the starting materials and ceramics obtained) were studied by X-ray phase analysis. The ceramic samples had the compositions corresponding to natural granite and tuff with additions of REE phosphates.
Microprobe analysis (on a Camebax instrument) was used to determine the compositions of the starting materials and products of experiments. The results are presented in Tables 1 and 2.
Table 1. Chemical composition of the starting rocks
Rock |
Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | FeO |
| Granite Le2 | 2.89 | - | 14.69 | 74.58 | 5.67 | 0.23 | 0.59 | 1.43 |
| Granite Le7 | 3.37 | - | 13.70 | 77.00 | 4.77 | 0.17 | 0.61 | 1.12 |
| Tuff 211A | 2.59 | 7.71 | 22.53 | 51.41 | 0.02 | 15.27 | 0.03 | 6.38 |
Table 2. Chemical composition of ceramics obtained
N |
Composition | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | FeO | Ce2O3 | P2O5 | La2O3 | SrO |
| Fm1 | LaPO4+Le2 | 4.48 | - | 12.38 | 66.78 | 4.03 | - | 1.14 | - | 5.21 | 5.47 | 0.43 |
| Fm2 | LaPO4+Le7 | 5.43 | - | 10.20 | 61.06 | 4.04 | - | 0.95 | - | 7.78 | 10.08 | 0.41 |
| Fm3 | CePO4+Le2 | 1.11 | - | 6.10 | 34.87 | 2.36 | 0.22 | 0.56 | 36.07 | 18.17 | - | 0.39 |
| Fm4 | CePO4+Le7 | 0.87 | - | 5.96 | 26.60 | 1.58 | 3.00 | 0.39 | 39.17 | 22.10 | - | 0.19 |
| Fm5 | CePO4+.Tuff | 1.36 | 1.93 | 12.35 | 29.45 | 0.44 | 5.84 | 2.03 | 30.57 | 15.62 | - | - |
| Fm7 | CePO4+Le7 | 2.64 | - | 8.58 | 31.96 | 3.46 | 0.42 | 1.05 | 25.41 | 13.26 | - | - |
| Fm8 | CePO4+Tuff | 2.28 | 3.18 | 11.26 | 29.68 | 0.50 | 5.71 | 5.65 | 27.27 | 13.81 | - | - |
Table 3. Leaching rates V (g/m2.days) of elements from ceramic samples
| Sample no | Density, g/cm2; | Leaching element | Wt.% in starting sample | 0-1 days | 1-7 days | 7-14 days | 7-28 days |
| Fm1+LaPO4 + + granite Le2 |
1.91 | Al P La Na |
5.56 2.94 4.65 3.32 |
0.890 3.300 0.079 7.802 |
0.15 1.260 0.025 2.030 |
0.050 0.400 0.078 0.580 |
0.022 0.230 0.008 0.386 |
| Fm2+LaPO4 + + granite Le7 |
1.95 | Al P La Na |
5.41 4.39 8.54 4.02 |
1.020 1.540 0.079 - |
0.240 0.650 0.010 0.991 |
0.056 0.180 0.009 0.352 |
0.020 1.103 0.009 0.191 |
| Fm3+CePO4 + + granite Le2 |
1.90 | Al P Ce Na |
3.23 10.24 30.66 0.82 |
0.280 0.015 0.029 - |
0.170 - 0.005 0.140 |
- 0.012 0.005 0.050 |
0.001 0.048 0.003 0.022 |
| Fm4+CePO4 + + granite Le7 |
2.24 | Al P Ce Na |
3.16 12.16 33.29 0.64 |
0.304 0.009 0.183 - |
0.110 0.148 0.004 - |
0.004 - 0.002 - |
0.002 0.006 0.002 - |
| Fm5+CePO4 + + tuff |
2.05 | Al P Ce Na |
6.55 8.31 24.58 1.00 |
0.220 0.220 0.013 - |
0.103 0.075 0.004 0.186 |
0.005 0.006 0.003 0.067 |
0.004 - 0.003 0.029 |
79
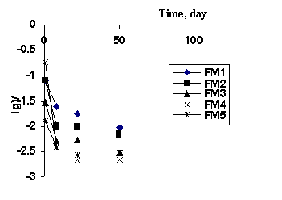
Fig.1. Kinetics of leaching of La and Ce from ceramic samples.
Fig. 2. Kinetics of leaching of phosphorus from ceramic samples.
Determination of leaching rate. The quality of ceramics was estimated from the leaching rates of elements from the samples in distilled water at 90oC (MAGATE MSS-1 test [4]). The results of the experiments on leaching of elements from the ceramic samples are presented in table 3 and Figs. 1 and 2.
Results and discussion. As can be seen in tables and plots presenting the results of experiments on leaching, the samples studied bind elements by ceramization at 1040-1180oC of the mixtures of their precipitated concentrates with natural mountain rocks.
The data on leaching of phosphate-silicate ceramic matrices obtained in the first series of experiments demonstrated their sufficiently high stability despite the low density. The analysis of results indicates that the leaching rate in the first 8 days of testing has the maximum value for all samples. During this period, only 0.002-0.015% lanthanum and 0.001-0.008% cerium contained in the sample are transferred to the leaching medium. The denser samples of the second series (2.74 and 2.89%) allow one to expect lower leaching rates.
After 50-day exposure under MSS-1 test conditions, for the granite-based samples, the mean leaching rate of cerium was 2.78x10-3 g/m2 day, and that of lanthanum was 8.24x10-3 g/m2 day, which is comparable with the leaching rates of elements from synroc samples [5]. The predomination of the leaching rate of phosphorus over that of REE allows one to consider that REE are bound in structures of newly formed minerals.
References:
Dunaeva A.N. Calculation of ion-exchange equilibria constants at modeling of 137Cs sorption in water-containing natural systems.
key words [Cs sorption model calculation water] GEOKHI
80
The empiric partitioning coefficients in the water-rock system (Kd) are extensively employed in modelling the migration of radionuclides in ground waters within selected contaminated territories. It is, however, evident that the field of usage of these coefficients cannot be extended into regions with other indices of the componental composition of grounds and/or natural waters.
The approach based on thermodynamic modelling of the competing sorption of toxic elements by a polymineral ground from a multicomponent aqueous solution is, in our opinion, more universal for the estimation of radionuclides partitioning in the water-rock system. Such modelling is based on the knowledge of constants of radionuclides sorption by individual minerals.
The goal of the work is 1) to obtain constants of the ion exchange of cesium and macrocations K+, Ca2+, Na+, Mg2+ on kaolinite, montmorillonite, illite, and halloysite on the base of processing of the experimental sorption isotherms. 2) Using the obtained data to show the possibility of radionuclide sorption from a multicomponent solution by a polymineral rock.
Theoretical basis. The thermodynamic processing of the experimental data is based on the idea of sorption as reaction of ionic exchange between the dissolved material and mineral-sorbent. It is assumed herewith that only the part of the mineral complying with its sorption capacitance is reactable. So, formally, the sorption process can be expressed by the following chemical reaction:
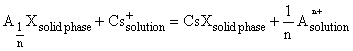 , where An+ is an ion-exchange cation of the mineral-sorbent, n is the charge of this cation, X is the anion component of the mineral. The reaction constant with allowance for the exhaust of the sorption capacitance is written as
, where An+ is an ion-exchange cation of the mineral-sorbent, n is the charge of this cation, X is the anion component of the mineral. The reaction constant with allowance for the exhaust of the sorption capacitance is written as
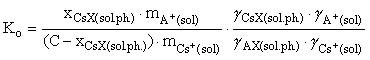 (1)
(1)
where x, m, and 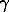 are the concentrations, molalities, and coefficients of component activity of the system, respectively, C is the sorption capacitance of the mineral, considered with respect to cesium being adsorbed as ideal solid solution. The molalities and coefficients of ionic activity in an aqueous solution were calculated with allowance for possible hydrolysis and complex formation processes in the water phase using the Debye-Hückel theory (third approximation).
are the concentrations, molalities, and coefficients of component activity of the system, respectively, C is the sorption capacitance of the mineral, considered with respect to cesium being adsorbed as ideal solid solution. The molalities and coefficients of ionic activity in an aqueous solution were calculated with allowance for possible hydrolysis and complex formation processes in the water phase using the Debye-Hückel theory (third approximation).
The concentration of the sorbed cesium in mineral for each I-th experimental point of the sorption isotherm is written as
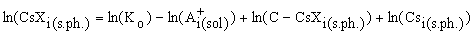 (2)
(2)
The equation (2) is used to approximate the experimental sorption isotherm. In this case the function 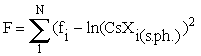 , where N is the number of the runs, fi is the concentration of cesium being adsorbed in the run minimised relative to two unknowns: the constants of the sorption reaction, Ko, and the sorption capacitance of the mineral, C.
, where N is the number of the runs, fi is the concentration of cesium being adsorbed in the run minimised relative to two unknowns: the constants of the sorption reaction, Ko, and the sorption capacitance of the mineral, C.
Processing of the experimental data. The technique described was used to process the experimental sorption isotherms of Cs on K, Na, Ca, Mg montmorillonites and halloysites and kaolinites enriched in these cations, as well as the data on Cs sorption on K- and Na-enriched illites (Whalberg J.S., 1962). The experiments were run from chloride solutions on macrocations at various concentrations of the competing ion (from 0.002 to 0.2N) in the range of cesium contents in water from 10-10 to 0.1 mole/l.
The values of Ko and C derived from the experimental data processing made it possible to numerically reproduce the experimental sorption isotherms (fig.1).
Fig.1. Experimental (Whalberg J.S., 1962) and modelling sorption isotherms of cesium by clay mineral. Cesium concentrations in solid phase and in water solution are in mole/kg and mole/l respectively. ▲,+,◊-experimental data; calculation curves.
On the base of the obtained thermodynamic information, using the equilibrium calculation program SlgSol (Mironenko M.V., 1995) we modelled sorption of cesium from chloride and nitrate solutions of various concentrations in the other experimentally studied simple systems. The results of the thermodynamic calculation are shown in fig.2 in comparison with the experimental data (Comans R.N.J., 1990 and Brouer E., 1983).
Fig.2. Experimental data of cesium sorption on K-saturated illite (Comans R.N.J., 1990 and Brouer E., 1983) in comparison with the thermodynamic calculations carrying on the basis of constants, obtained in the present work from Whalberg J.S., 1962 experiments. Cesium concentration in solid and solution is represented in mole/kg and mole/l respectively. ●■-experimental data Bover E., 1983; ∆-experimental data Comans R.N.J., 1990; calculation isotherms.
81
Modelling of competing sorption of cesium in the polymineral rock-multicomponental water solution. The processing of the derived constants of cesium sorption by the Hess law made it possible to calculate the constants of the competing sorption of K+, Ca2+, Na+, Mg2+, Cs2+ on said minerals. This enabled modelling of cesium sorption from water solutions, containing basic cations of natural waters, on polyminerals rocks. Fig.3 exemplifies the calculation of cesium, sorption, using the program SlgSol, from the solution containing K+, Ca2+, Na+, Mg2+, Cs2+ by the ground that consists in equal proportions of illite, kaolinite, halloysite, and montmorillonite. It has been shown that not only the value of the total sorption of cesium by the ground as a whole but, also, the contribution of each mineral - the ground constituent can be estimated.
Fig.3. Results of thermodynamic calculation of cesium sorption from multicomponent water solution by polymineral soil.
In our opinion the creation of the database on sorption of radionuclides and heavy metals by minerals will make it possible to model their behaviour in the systems composed of polymineral rocks and solutions of various geochemical composition.
References:
82