Methods and experimental technique
Zhdanov N. N., Osadchii E.G., and Lunin S. E. Modern apparatus for investigation in experimental geochemistry (new instruments for measuring electrochemical potentials, temperature, and pressure, and for temperature control).
key words[modern apparatus experimental geochemistry]
The appearance of precision, micropower, polyfunctional electronic instruments in the market of electronic production gives a possibility to develop laboratory and field measuring apparatus corresponding to requirement of modern experiment. Analog-to-digital converters (A/D), digital-to-analog converters (D/T), stable and accurate sources of reference voltage, etc. are related to these components. A microprocessor, through which information is counted and transferred to a computer, is the main unit controlling the work of these electronic microcircuits.
The following instruments for studying natural objects and performing laboratory experiments were developed and produced in IEM RAS. The hydrochemical high-temperature probe with high-ohmic input amplifiers to 12 GOhm and schemes for switching-on pressure and temperature detectors is designed for measuring electrochemical potentials, temperature, and pressure in sea media at a depth to 10 km and in natural hydrothermal sources. In ecology it can be used for monitoring natural water reservoirs.
The combined laboratory measuring instrument with four high-ohmic inputs for switching-on electrochemical detectors, six special inputs for switching-on thermocouples, and four inputs for switching-on sapphire pressure detectors of the D-100 type. In these instruments, the necessary channels are chosen and measured information is collected through a computer via the successive channel in record RS-232.
The computer-controlled precision temperature controller makes it possible to establish the temperature of an object by any specified program, for example, in DTA measurements where a rigidly linear temperature change is required or to bring electrochemical cells into the regime where the rate of temperature change should not exceed some value.
These systems allow one to automate experiments related to achievement of equilibrium of a certain parameter, for example, potential in electrochemical measurements, and at constant temperature, the "temperature titration method." The achievement of equilibrium is determined in the automated regime. Then the measurement is performed, and the computer program establishes the next temperature and goes to the measurement regime to analyze the data obtained for determining the achievement of the next equilibrium.
The packet of special programs, which allow one to visualize the process of experiment in the regime of real time, was developed. The data obtained from any channels can be written in different files during experiment. There is a possibility to choose experimental points, average them, and write in a special file for subsequent processing by standard graphic programs. All data are stored in text files, which can be easily read. This allows a researcher who knows a simple programming language of high level, for example, BASIC, to write a necessary program for processing the results obtained.
Shornikov S.I., Archakov I.Yu., and Natalenko P.G. Ion source for a MI-1201 mass spectrometer for high-temperature studies.
key words [mass spectrometer ion source]
The study of high-temperature properties of refractory materials, in particular, processes of evaporation at temperatures higher than 1500 K, is one of the priority directions of the modern materials science. The combination of the effusion method and mass spectrometric analysis of the gas phase composition is the most information-bearing method for studying these processes. As known, specialized mass spectrometers for these purposes (for example, MS-1301) are produced in insignificant amounts by few industrial companies. Therefore, mass spectrometers for general purposes are mostly often modified for studies at high temperatures.
In this work, we attempted to develop the corresponding attachment for the most popular series MI-1201 mass spectrometer.
The ion source for high-temperature studies was designed and prepared on the basis of a typical gas source used in a MI-1201 mass spectrometer. The possibility of heating the effusion chamber to 3000 K at the total pressure of residual gases not higher than 10-11 atm is the distinctive feature of this structure from those suggested previously [1-3].
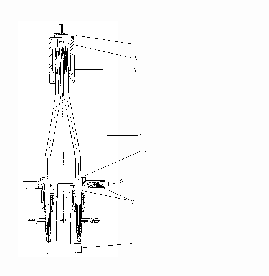
Fig. 1. High-temperature heater for the ion source. Designations: 1- shutter overlapping the molecular beam; 2- effusion chamber; 3- water-cooling jacket; 4- copper cooling current leads; 5- flange of the heater; 6- current leads of the thermocouple; 7- teflon seals; 8- flange of the pyrometric window.
61
The ion-optical system of the source does not differ basically from the typical one. The scheme of the heater is presented in Fig.1. The effusion cylindrical chamber is prepared of the refractory metal foil and fixed in water-cooling current leads. Unlike traditional methods of heating effusion chambers (electron bombardment or radiation heating), in this case, the effusion chamber is heated by passage of the electric current (power 600 W at currents to 200 A) through the chamber. This method for heating the effusion chamber has been previously suggested in [4]. The structure suggested makes it possible to decrease substantially the thermal inertia of the chamber and its gas recovery, which allows an increase in the temperature interval of studies as compared to the structures described previously. It is noteworthy that a decrease in gas recovery allows the standard system of pumping out to be used without using additional diffuse pumps at sufficiently high heating velocities of the chamber.
The temperature of heating of the effusion chamber can be measured by both a thermocouple (to 2200 K) and an optical pyrometer through a window of pyrometric visualization arranged axially to the flange of the ion source. Since the geometric parameters of the chamber without additional attachments do not allow one to perform the regime of imitation of the "absolutely black body" radiation, a correction related to the surface radiation should be introduced when the heating temperature of the chamber is determined by the optical pyrometer.
In the temperature range not higher than 2200 K, indications of the pyrometer can be corrected by the indications of the thermocouple, and at higher temperatures, at calibration of the pyrometer indications, by reference temperature points (melting points of standard substances).
References:
#This work was financially supported by the Russian Foundation for Basic Research (Project No. 97-03-33414a).
62