Isotopy
Reutskii V.N., Borzdov Yu.M. Fractionation of carbon isotopes at crystallization of diamond and graphite using a temperature gradient technique.
key words [carbon isotopy diamond graphite]
Fractionation of carbon in a model melt-carbon system is a matter of interest to researchers concerned with the problems of carbon geochemistry related with the problem of diamond formation. Diamond crystallization experiments enable one to study the behavior of isotopes under controlled conditions. The earlier studies showed that at high-rate short-term synthesis of diamond from graphite no significant alteration occurs in the isotopic composition of the initial and end phases [1]. The authors of [2] report weighting of diamond's carbon against the initial one at the gas-phase crystallization.
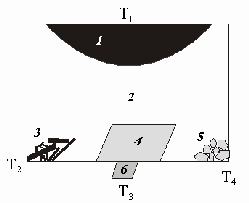
Fig. 1. Schematic arrangement of the phases in the reaction volume. 1 - carbon source; 2 carbon dissolved in the Fe-Ni melt volume; 3 graphite crystals; 4 diamond on a seed; 5 spontaneous diamond crystals; 6 seed crystal. “1 temperature in the dissolution zone; “2, “3, “4 temperatures in the growth zone; “1>T2>T3T4.
62
A number of the runs devoted to carbon crystallization in the Ni-Fe-C were carried out at P=55kbar T=1450oC for 40 h using the technique of [3]. The carbon source was graphite MG-1. In order to study the fractionation of isotopes between the carbon phases the experiment was performed wherein joint crystallization of diamond and graphite was observed (fig.1). As a result the following carbon phases were produced and studied: carbon source (graphite that remained undissolved); fine-crystalline carbon (FCC); graphite crystals; diamond on a seed and spontaneous diamond crystals. The FCC forms at a 'quenched' cooling of a taenite melt. Carbon was transformed to CO2 on an apparatus intentionally desighned for working with small amounts of gas under high vacuum conditions. The charge (1mg) packed into a platinum capsule was oxidied in a quartz reactor at 900oC for 20 min. Oxygen was generated by heating CuO to 850oC. The isotopic ratio was measured on a mass-spectrometer Finnigan-MAT Delta, the inaccuracy not exceeding 0.02%o. The obtained data (table 1) suggest that the synthesized diamond and the initial graphite are close in the isotopic composition of carbon. Graphite crystals are quite enriched in 13C isotopes. The FCC has a smaller amount of isotopes both with respect the rest of the carbon-containing phases and the initial graphite. The reproducibility of the isotopic analysis results for diamonds (n=6), graphite crystals (n=3), and carbon source (n=3), including the carbon oxidation procedure, was 0.2%o, for the FCC - 0.3%o (n=3), and for the initial graphite MG-1 - 0.4%o (n=4). The inidentical reproducibility of the analysis for individual phases indicates the different homogeneity degree of the analyzed materials.
Table 1. Isotopic composition of various carbon phases
Sample |
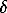 13C, %o PDB 13C, %o PDB |
+ |
Carbon source |
-28.0 |
0.2 |
Fine-crystalline carbon |
-31.3 |
0.3 |
Graphite crystals |
-26.6 |
0.2 |
Diamond on a seed |
-28.2 |
0.2 |
Spontaneous diamond crystals |
-27.9 |
0.2 |
Graphite |
-27.8 |
0.4 |
Fractionation of carbon isotopes in the experiment in question could have place both at the crystallization stage, between carbon, dissolved in a melt, graphite and diamond, and upon the system's cooling, between FCC, carbon solid solution in taenite and a fluid. The determination of the isotopic composition of carbon contained in the two latter phases is of considerable methodic difficulties and has not been performed at this stage of work.
According to the theoretic calculation [4], under equilibrium conditions at temperatures from 300 to 1500oC and pressures in excess of 20 kbar the isotopic fractionation between graphite and diamond occurs towards the 13C enrichment of graphite. It was found in [5] that disseminated carbon in graphite eclogite from the kimberlite pipe 'Mir' is quite lightened in the isotopic composition (d 13C = -25.19 PDB) with respect to carbonate and graphite from the same sample (d 13C = -9.9; -4.5 PDB, respectively). The authors of [6] also report the 12C isotope enrichment of carbon disseminated in igneous rocks. The isotopic fractionation between the phases in experiment agrees well with the regularities revealed in studies of natural objects associated with the diamond formation.
So, during the growth of diamond and graphite by the temperature gradient technique isotope fractionation between carbon phases, in this case, the isotopic composition of diamond corresponds to d 13C of the initial graphite. Graphite, crystallizing together with diamond is relatively enriched in 13C isotope. The isotopic composition of carbon dissolved in a melt is significantly enlightened with respect to other phases and the initial graphite.
References:
#Ustinov N.I., Ul'yanov A.A. Intrastructural partitioning of oxygen isotopes in silicates with a layered structure: estimation of the lomonosovite-murmanite transition conditions.
key words [oxygen isotope lomonosovite murmanite]
The identity of the 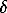 18O values of the silicon-oxygen frameworks of the sequentially forming silicates with a layered structure can be a direct evidence for the supposed mineral transformation without a significant framework structure reorganization.
18O values of the silicon-oxygen frameworks of the sequentially forming silicates with a layered structure can be a direct evidence for the supposed mineral transformation without a significant framework structure reorganization.
In this aspect in the case of silicophosphates noteworthy is the hypothesis that lomonosovite is the protimineral for murmanite the formation of which is, posssibly, associated with the alteration of lomonosovite under the action of hydrothermal solutions or surface waters [Minerals. Reference book, V.III, is.I. M. Nauka, 1972]. The study of the character of the oxygen isotopes partitioning for this mineral pair is based on the difference in the chemical parameters of the oxygen isotopic exchange with water of SiO4 and PO4 anions. We selected several specimens of lomonosovite for our study, but the necessary criteria with respect to purity were possessed by three specimens (two from the Rasvumchorr Mountain and one from the Jukspor Mountain, the Khibiny alkali massif, Kola peninsula, Russia). Along with the initial lomonosovite specimens, the products of its laboratory decomposition (treatments in water and acids, thermal dehydration) and, also, murmanite mineral (from alkali pegmatites of the Lovozerosky massif, Kola penins., Russia) were subjected to oxygen-isotopic analysis. The selection of the specimens was dictated by the structural analogy of the lomonosovite and murmanite frameworks: the base is three-layer packets consisting of NaO6-, TiO6- and MnO6- octahedra and isolated Si2O7 groups; in the lomonosovite structure there are sodium-phosphate networks between the packets, in murmanite these networks are fixed by H2O molecules. If the process of leaching of sodium-phosphate layers from the lomonosovite structure and the incorporation of H2O molecules took place under the surface conditions (i.e. at low temperatures) then by assuming that there is no oxygen-isotopic exchange between the water and diorthogroups, one can expect the identity in the isotopic composition of the silicon-oxygen framework of lomonosovite and murmanite.
# This work is supported by the Russian Foundation for Basic Research (project N 97-05-64566).
63
At acidic treatment of lomonosovite for 30 min atT=100oC:
Na4Ti4Si4O18.2Na3PO4+HCl  SiO2
SiO2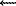 + 2PO42- (1)
+ 2PO42- (1)
A phosphate ion retains the initial isotopic composition of oxygen of the phosphate component therefore in accord with the balance calculation one can define the 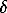 18O value of the silicon-oxygen framework of lomonosovite: the comparison of the oxygen-isotopic characteristics of the framework and the silicon formed by the reaction (1) makes it possible to judge about the isotopic effects at the restructuring of the lomonosovite silicate component (table).
18O value of the silicon-oxygen framework of lomonosovite: the comparison of the oxygen-isotopic characteristics of the framework and the silicon formed by the reaction (1) makes it possible to judge about the isotopic effects at the restructuring of the lomonosovite silicate component (table).
The modelling of the hydrothermal lomonosovite-to-murmanite transition (100oC, 24 h):
Na4Ti4Si4O18.2Na3PO4+nH2O 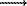 Na4Ti4Si4O18.nH2O +
Na4Ti4Si4O18.nH2O +
+ 2Na3PO4 (2)
has shown that the isotopic composition of the oxygen framework of the neogenic murmanite for the two studied specimens is in full correspondence with the result of the oxygen isotopic exchange framework-H2O (table). Therefore the lomonosovite -to-murmanite transition at elevated temperatures erases the isotopic 'labelling' of the parental structure. At the same time at a short acidic treatment silica is practically similar in the isotopic composition to the initial lomonosovite framework, which convincingly suggests that the rate of the structural 'transformation' of the silicate component of lomonosovite is considerably higher than that of the oxygen isotopic exchange of the formed silica and water.
So, the main conclusion of this study, namely, the fact of identity of the 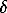 18O values of the lomonosovite and murmanite frameworks, can be interpreted as a consequence of the mineral lomonosovite-murmanite 'transition' only under low temperature conditions.
18O values of the lomonosovite and murmanite frameworks, can be interpreted as a consequence of the mineral lomonosovite-murmanite 'transition' only under low temperature conditions.
Table. Isotopic composition of oxygen of silicon-oxygen frameworks of lomonosovite and murmanite (% with respect to SMOW)
Mineral |
Initial composition from the balance calculation |
by react. (1) |
by react. (2) |
Lomonosovite |
7.3 |
-1.5 |
6.7 |
Murmanite |
7.9 |
- |
- |
Lomonosovite |
6.0 |
-0.5 |
6.5 |
Note: The initial specimens of lomonosovites and murmanite were characterized by the values 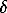 18O +4.3, +3.7, +2.6, phosphate residues 1.1 and -2.6, respectively.
18O +4.3, +3.7, +2.6, phosphate residues 1.1 and -2.6, respectively.
#Ustinov N.I., Ul'yanov A.A. Experimental oxygen-isotope study of the dehydration processes in serpentines.
key words [oxygen isotope serpentine dehydration]1-GEOKHI 2-Geological Depat. Of MGU
The determination of the oxygen-isotope shift  18O between the silicate framework and hydroxyl group of serpentine is a burning problem for isotopic geochemistry and mineralogy both in view of designing a monomineral isotopic geothermometer and fixing the scale and character of fractionation of oxygen isotopes in the process of transformation of minerals under the natural conditions.
18O between the silicate framework and hydroxyl group of serpentine is a burning problem for isotopic geochemistry and mineralogy both in view of designing a monomineral isotopic geothermometer and fixing the scale and character of fractionation of oxygen isotopes in the process of transformation of minerals under the natural conditions.
The oxygen-isotope analysis of mineral serpentine-magnetite and serpentine-calcite pairs from the Bazhenov chrysotile-asbestos deposit in the Karabash massif (South Urals ) and from garnet peridotite xenolite (Yakutia) enabled us to determine the parageneses temperature to be 320, 380, and 160oC, respectively for the samples under study.
The techniques for separating the material for intrastructural measurements were worked out in two lines: (1) short-time high temperature heating of the initial serpentine sample in vacuum ('impulse' dehydration) and (2) dissolution of serpentine in a mixture of hot acids (HCl+HNO3) and separation of the silica framework.
The impulse vacuum heating of serpentines leads to the complete dehydration of the latters which is unambiguously proved by the thermal analysis and IR-spectroscopy data. The restite after the acidic treatment of serpentine is significantly enriched in SiO2 and Al2O3, as compared with the initial sample and depleted in MgO and FeO. The atomic Si/Mg ratio ( in the first approximation proportional to the relationship between neogenic silica and nondecompresed serpentine) changes by more than a factor of 100: from 0.70 (theoretical value is 0.67) to 72.1. The two-hour boiling of the +0.05-0.25 mm fraction in a HCl+HNO3 mixture leads to almost 95% decomposition of serpentine (its relics in the restite were fixed only by methods of thermal analysis and IR-spectroscopy). As shown by the electron-probe microanalysis data, for larger fractions (+0.05-0.5 mm) leaching of Mg is incomplete, and the complete decomposition of serpentine necessitates longer times of the acidic treatment. The results of oxygen isotopic composition study in serpentines, products of their thermal and acidic decomposition are illustrated in fig.1 The magnitude of the intrastructural effect was determined as  18O=
18O=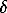 18Oserpentine-
18Oserpentine-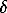 18OOH-group >and the graphs
18OOH-group >and the graphs  18O=f(T) were plotted on the base of the above 'isotopic' temperatural estimations.
18O=f(T) were plotted on the base of the above 'isotopic' temperatural estimations.
# This work is supported by the Russian Foundation for Basic Research (project N 97-05-64566).
64
The fact of a considerable difference in the isotopic data for the two series of measurements (series I 'impulse' degassing, series II-acidic treatment) was unexpected. The main feature of the results of the investigations is, herewith, the regular parallelity of the 'straight' lines of for the two run types. Such a position can only by treated in the case that the conditions for running the serpentine dehydration experiments are stable. The deviation of the 'acidic series' 'straight line' towards 'light' side is due to a partial oxygen-isotope exchange between the forming silica and hydrous acidic solutions: Mg3Si2O5(OH)4 + 6HCl 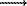 2SiO2 + 5H2O +3MgCl2 (100oC, 30 min). In this case an increase in the acidic treatment duration leads to a regular decrease of the
2SiO2 + 5H2O +3MgCl2 (100oC, 30 min). In this case an increase in the acidic treatment duration leads to a regular decrease of the 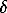 18O () value for one of the serpentine samples: 9.9 (2h), 8.2 (4h), and 4.0 (10h). The above mentioned together with analogous behaviour of the 'straight' line (III)
18O () value for one of the serpentine samples: 9.9 (2h), 8.2 (4h), and 4.0 (10h). The above mentioned together with analogous behaviour of the 'straight' line (III)  18O=f(T) (fig.1) for talc (thermal dehydration) [Ustinov V.I., Ul'yanov A.A. Geokhim. 1996, N8, 791-795] suggest that once the experimental conditions are stable, the obtained results can be employed for the estimation of the temperatural regime of the serpentines formation.
18O=f(T) (fig.1) for talc (thermal dehydration) [Ustinov V.I., Ul'yanov A.A. Geokhim. 1996, N8, 791-795] suggest that once the experimental conditions are stable, the obtained results can be employed for the estimation of the temperatural regime of the serpentines formation.
It also seems promising to use much thinner serpentine fractions (0.01 mm) and a cold acidic treatment of the initial samples for a complete elimination of the isotope-exchange reactions.
65