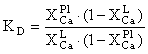
KHITARIADA-98
Magmatic systems, fluid-magmatic interaction, melts properties
#Litvin V.Yu., Gasparik T., Litvin Yu.A., Bobrov A.V. Melting experiments on the En-Ne and Fo-Jd joins at pressures 6.5-13.5 GPa.
key words [alkaline magmatism pressure mantle Na2Mg2Si2O7]
Alkaline magmatism is an intriguing problem of the Earth's mantle petrology. High-pressure experiments on the Fo-Jd [1, 2] revealed the reaction between forsterite and jadeite giving rise to a new Na-Mg-silicate phase Na2Mg2Si2O7 (NMS) and pyrope Mg3Al2Si3O12. It was suggested that NMS-phase could be presented in the Ne-normative mantle composition. It was also proposed that this plausible mantle mineral plays an important role in solidus mineral assembly of the alkaline mantle as well as for generation of primary alkaline magmas.
The enstatite(En)-nepheline(Ne) join connects olivine(Ol)-normative and Ne-normative mantle compositions in the base ternary system Fo - (En) - SiO2 - (Jd) - Ne. The En-Ne join intersects Fo-Jd one as well.
Table 1. Conditions of melting experiments on the En-Ne join at 6.5 GPa and compositions of phases
| Run N | Composition, mol % | To C | Duration of experiment, min | Phases | Cations per 24 oxigen Na Mg Al Si | Stable assemblages |
| 175 | 80/20 | 1500 | 10 | Jd | 3.233 0.685 5.322 6.858 |
Fo+Jd |
| 182 | 80/20 | 1600 | 15 | Fo Jd L |
- 11.855 - 6.073 3.720 0.657 4.059 7.698 3.344 3.003 2.825 7.544 |
Fo+Jd+L* |
| 173 | 70/30 | 1600 | 5 | Ga Fo L L L |
0.509 5.225 3.880 6.350 0.204 11.600 0.330 6.124 4.204 2.450 2.606 7.770 2.730 2.472 2.870 7.929 3.739 2.181 3.094 7.654 |
Fo+Jd+Py+L* |
| 176 | 40/60 | 1500 | 10 | Jd NMS |
3.017 1.044 5.402 6.672 6.853 6.610 0.154 6.686 |
Jd+NMS+Py? |
Experimental studies of the En-Ne and Fo-Jd joins were carried out using a high-pressure apparatus 'anvil with hole' at 6.5 GPa (at the Institute of Experimental Mineralogy, Chernogolovka, Russia) and an uniaxial split-sphere apparatus USSA-2000 in the 12.5-13.5 GPa pressure range (at the State University of New York in Stony Brook, U.S.A.).
Melting relations on the En-Ne join were investigated at 6.5 GPa in the 1400-1700oC temperature interval. The experimental conditions and phase compositions are given in Table 1.
The major liquidus phases of the En - Ne pseudobinary join are jadeite solid solution (Jdss) and En, the latter is replaced by Fo in the Mg-rich part of the system. Peritectic relations were observed on the solidus. The result of the peritectic reaction is the disappearance of Fo from the crystallising assemblage. En-Ne join is unstable under high pressures and major phases of the low-temperature subsolidus assemblage are Fo and Jd. Increasing the temperature up to 1500oC gives rise to the reaction between Fo and Jd producing NMS, Py and Jd with a sensible content of MgO. The garnet phase in the experimental products was observed just once in the course of microprobe analysis, but the X-ray diffraction method showed its presence permanently. Apparently that might be explained by kinetics difficulty with garnet nucleation. At the same time, garnet is one of the major crystallising phases in the Fo-Jd join studied at 6.5 GPa in similar experimental and composition conditions.
Experimental data for the En-Ne join investigated in the 12.5-13.5 GPa pressure range are given in Table 2.
The quenched mineral assemblies consist of Fo, jadeitic Cpx, clinoenstatite modification of MgSiO3 (CEn), Ga and easily fusible phase NMS. Na-rich glasses interpreted as quenched solidus melts were observed as well. Quenched melt obtained in run N2989 (Table 2) is extremely enriched in Na and thus might be estimated as the first melt which appears on the solidus of the system. Experiments at 12.5-13.0 GPa and 1850oC revealed the subsolidus field including Fo, Cpx, Ga and CEn. The appearance of NMS was observed at pressures 13.0 -13.5 GPa. The subsolidus conditions in this new NMS-bearing assemblage were stable only at 1750oC. Increasing the temperature caused the appearance of the melt.
5
Table 2. Conditions of melting experiments on the En-Ne join at 12.5-13.5 GPa and the average compositions of phases
| Run N | Compo - sition mol % | P, GPa | T, C | Duration of experiment, hours |
Phases | Na2O weight % | MgO weight % | Al2O3 weight % | SiO2 weight % | Sum |
| 2807 | 70/30 | 13.5 | 1700 | 5 | Ga CEn Fo NMS |
3.15 0.66 0.18 20.7 |
28.20 37.87 55.88 28.76 |
14.16 0.65 0.33 1.55 |
53.65 59.34 42.3 55.46 |
99.15 98.5 98.7 97.01 |
| 2989 | 70/30 | 13.5 | 1850 | 0.5 | Ga NMS L |
3.10 23.32 36.91 |
27.46 27.08 15.66 |
17.92 2.93 4.35 |
52.74 47.05 48.22 |
101.21 100.37 105.13 |
| 2814 | 70/30 | 13.0 | 1700 | 5 | Ga Cpx Fo |
2.94 7.44 0.18 |
28.57 22.23 56.74 |
14.12 8.33 0.22 |
54.21 61.58 42.73 |
99.84 99.58 99.88 |
| 3011 | 70/30 | 13.0 | 1850 | 0.5 | Ga NMS L |
3.71 23.21 15.74 |
26.85 28.19 15.01 |
17.73 2.59 13.10 |
53.70 46.72 48.55 |
101.99 100.71 92.4 |
| 3026 | 80/20 | 13.0 | 1850 | 0.5 | Ga Cpx Fo |
2.49 6.25 0.15 |
27.79 24.49 55.71 |
19.41 7.61 0.30 |
49.79 60.71 42.08 |
99.47 98.53 98.23 |
| 3017 | 80/20 | 12.5 | 1850 | 0.5 | Ga Cpx CEn Fo |
1.74 4.74 0.64 0.14 |
29.66 27.81 38.16 55.88 |
17.94 6.94 1.02 0.30 |
51.50 59.83 59.94 43.28 |
100.84 99.34 99.77 99.6 |
| 3023 | 80/20 | 12.7 | 1750 | 0.5 | Ga Cpx CEn NMS |
2.51 6.29 0.48 14.88 |
27.26 24.62 39.21 32.72 |
20.97 8.70 0.76 1.92 |
49.88 59.84 59.92 49.33 |
100.62 99.45 100.37 98.85 |
Thus, the temperature of the solidus of the En-Ne join is controlled by melting of NMS, which appearance in subsolidus assemblage causes the sharp decrease of the solidus temperature at pressure range from 13.0 to 13.5 GPa.
Conclusions:
1. Peritectic relations on the solidus of the pseudo binary system En-Ne (4-component in terms of oxides) which connects Ol-normative compositions of the model peridotite with highly alkaline Ne-normative compositon are experimentally revealed. Appearance of the NMS-phase in the solidus assembly of the Ne-normative mantle is effective in primary generation of the melts with high alkali content. Further evolution of the primary magmatic melts under conditions of fractional crystallisation at the near-solidus temperatures leads to the further increase of the alkalinity of the magma and disappearance of Ol-phase from the crystallising assembly.
2. This study gave first experimental data testifying the possibility of sharp decrease of solidus temperature of the anhydrous Ne-normative mantle at 13.0-13.5 GPa with the appearance of NMS-phase in the subsolidus assembly. These data allow to offer a new hypothesis of the chemical nature of the 400 km discontinuity between upper mantle and transition zone if the high alkaline content in this region is suggested.
References:
6
#Matveev Yu.A., Litvin Yu.A., Perchuk L.L., Chudinovskikh L.T., Yapaskurt V.O. Experimental studies of the carbonate - silicate system K2Mg(CO3)2 - (Ca/Ca0.5Mg0.5)SiO3 - Al2O3 in connection with the problem of Kokchetav-type diamond genesis.
key words [carbonate-silicate reactions K-clinopyroxene pressure diamond]
Carbonate - silicate rocks from Kumdy Kul microdiamond deposits of Kokchetav metamorphic complex (northern Kazakhstan) contain potassium clinopyroxenes. The rocks are composed of isometric grains of dolomite (70%), garnet (Gros46Py40Alm12), clinopyroxene (Di, Hed) and amphibole. The mineral compositions are given in the table 1. Garnets contain numerous microinclusions of diamond (about 20 mk by size) and high potassium clinopyroxene (about 200 mk). It was found experimentally that the K-Al pair can substitute the Ca-Mg one in clinopyroxene structure at pressures above 6.0 GPa [1,2].
# This work is supported by the Russian Foundation for Basic Research (project N 98-05-64033) and Federal Programm "Integration" (project N 250).
6
Table 1. Compositions of natural minerals (Kokchetav metamorphic complex)
| Mineral | Oxides | ||||||||||
| SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | |||
Dol* |
0.18 | 0.00 | 0.13 | 4.25 | 0.69 | 36.17 | 57.91 | 0.62 | 0.04 | ||
Grt |
40.98 | 0.20 | 23.10 | 5.61 | 0.72 | 10.49 | 18.90 | 0.00 | 0.00 | ||
Grt |
40.35 | 0.09 | 22.78 | 12.26 | 1.61 | 9.78 | 12.95 | 0.17 | 0.00 | ||
Phl |
41.65 | 0.75 | 18.10 | 5.74 | 0.00 | 23.49 | 0.00 | 0.20 | 10.07 | ||
Cpx |
54.41 | 0.08 | 1.75 | 1.74 | 0.00 | 17.31 | 23.71 | 0.37 | 0.55 | ||
Cpx |
54.27 | 0.09 | 1.56 | 1.94 | 0.14 | 16.63 | 24.62 | 0.40 | 0.26 | ||
Cpx |
54.55 | 0.06 | 1.89 | 1.26 | 0.00 | 16.66 | 24.19 | 0.55 | 0.82 | ||
Amf |
51.26 | 0.35 | 10.47 | 4.35 | 0.10 | 19.01 | 13.05 | 0.42 | 1.00 | ||
Pl |
49.71 | 0.03 | 38.76 | 0.33 | 0.02 | 0.64 | 0.74 | 1.21 | 8.56 | ||
Note: Dol - dolomite, Grt - garnet, Phl - phlogopite, Cpx - clinopyroxene, Amf - amphibole, Pl - plagioclase.
Ultrahigh pressure minerals diamond and K-clinopyroxene seems to be formed as stable phases, and then were derived toward the Earth's surface by carbonate-rich liquids (or plastic materials). To confirm the proposed model, it should be clarified whether diamond and K-clinopyroxene would crystallise from carbonate-silicate immiscible liquids to form the mineral assembly observed in Kumdy Kul diamondiferous deposit. The system K2Mg(CO3)2 - CaSiO3 - Al2O3 which is chemically similar to the carbonate-silicate rocks mentioned above was chosen for experimental study of the problem. The ternary system K2Mg(CO3)2 (KMC) - CaSiO3 (Wol) - Al2O3 (Cor) consists of two simpler ternary subsystems KMC - Gros - Wol and KMC - Gros - Cor. The system K2Mg(CO3)2 - CaMgSi2O6 (Di) - Ca3Al2Si3O12 (Gros) was also involved in experimental investigation. Several compositions of the KMC - Gros - Cor, KMC - Wol - Gros and KMC - Di - Gros joins were studied at pressure 7 GPa within 1200 - 1700oC.
Experiments were performed using a high pressure apparatus of 'anvil-with-hall' type. The starting mixtures of synthetic K2Mg(CO3)2, CaSiO3, CaMgSi2O6, Ca3Al2Si3O12 and Al2O3 were placed in sealed Pt capsules.
Intensive carbonate-silicate reactions were revealed in the course of which spinel MgAl2O3, diopside and K-rich clinopyroxene, pyrope-grossularite garnet, forsterite, montichellite, phlogopite-like K-silicate, K-Al- silicate (is seldom), as well as K-, K-Ca- , Ca-, Mg-, Ca-Mg- carbonates formed. Silicate balls of garnet composition were found at higher temperatures indicating melting processes. The experimental conditions and some experimental results and phase compositions are presented in Table 2.
Table 2. Compositions of experimental phases
(the starting composition KMC50Di25Gros25)
Sample |
To C | Run duration,min | Oxides |
|||||
| SiO2 | Al2O3 | MgO | CaO | K2O | Phase | |||
| 166 | 1300 | 60 |
55.39 | 3.39 | 16.60 | 22.88 | 1.74 | Di |
| 55.07 | 6.65 | 13.87 | 19.37 | 4.83 | Di |
|||
* |
42.20 | 23.41 | 11.27 | 22.99 | 0.09 | Grt |
||
| 178 | 1000 | 75 |
42.65 | 23.14 | 12.27 | 21.60 | 0.09 | Grt |
| 55.18 | 0.09 | 17.91 | 26.05 | 0.56 | Di |
|||
| 156 | 1200 | 90 |
40.98 | 22.87 | 5.59 | 30.52 | 0.04 | Grt |
| 54.64 | 2.80 | 17.08 | 24.43 | 0.69 | Di |
|||
| 184 | 1600 | 15 |
41.72 | 23.28 | 6.19 | 28.64 | 0.14 | Grt |
| 52.00 | 8.55 | 12.12 | 23.87 | 2.93 | Di |
|||
Conclusions:
1. Some new high-pressure carbonate-silicate reactions in the K2Mg(CO3)2 - CaSiO3 (CaMgSi2O6) - Al2O3 system at 7 GPa and 1200-1700oC were established.
2. Potassium-rich clinopyroxene (K2O content is up to 5 wt. %) was identified among experimental products.
3. Experimental high-pressure assembly of carbonate and silicate minerals (garnet + K-rich diopside + Ca-Mg-carbonates) is similar to that of natural Kumdy Kul diamondferous rocks.
References:
Pletchov P.Yu. and Gerya T.V. Effect of H2O on plagioclase-melt equilibrium.
key words [plagioclase melt equilibrium magmatic crystallization.]
The plagioclase-melt equilibrium is well investigated in anhydrous systems (Drake, 1976; Ariskin, Barmina, 1990; Pajasawatwong et al., 1995). Many authors mention that an increase in the water content in the melt results in a considerable (by 20-40 numbers) shift of compositions of crystallizing plagioclases to a more Ca region (for example, Housh, Luhr, 1991; Danyshevsky et al., 1997).
This model is based on the exchange equilibrium of Ca and Na between co-existing plagioclase and melt under both dry and water-saturated conditions.
The exchange equilibrium constant (KD) is:
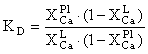
On the other hand, the equilibrium of the reaction is achieved when is minimum of the free Gibbs energy. The latter conditions can be presented in the simplified form as follows:
 Go +
Go +  GH2O + GeAn - GeAb = -RTln(KD), (1)
GH2O + GeAn - GeAb = -RTln(KD), (1)
where  Go=
Go=  H T
H T S + P
S + P V
V
 GH2O= ZH2O (
GH2O= ZH2O ( HH2O -
HH2O -  SH2OT +
SH2OT +  VH2OP), (2)
VH2OP), (2)
where ZH2O = XH2O/XH2O(sat)
The excessive energies for a solid solution of plagioclases (Perchuk et al., 1990) are:
7
GeAn = 2W21XAn(XAb)2 + W12.[(XAb)3 - XAn(XAb)2] (3)
GeAb = 2W12XAb(XAn)2 + W21.[(XAn)3 - XAb(XAn)2] (4)
W12= 1980-1.526T, W21= 6860-3.877T. (5)
We obtained the general equation for this reaction with unknown coefficients  H (reaction enthalpy),
H (reaction enthalpy),  S (reaction entropy),
S (reaction entropy),  V (volume effect of the reaction),
V (volume effect of the reaction),  HH2O,
HH2O,  SH2O, and
SH2O, and  VH2O (effect of water to enthalpy, entropy, and volume, respectively). The authors selected the following coefficients by the least-squares method from a set of 198 experimental melt-plagioclase pairs (INFOREX, Ariskin et al., 1997):
VH2O (effect of water to enthalpy, entropy, and volume, respectively). The authors selected the following coefficients by the least-squares method from a set of 198 experimental melt-plagioclase pairs (INFOREX, Ariskin et al., 1997):
|
|
|
|
|
|
This model describes only the exchange equilibrium of Na and Ca between plagioclase and melt and does not include the crystallization reaction of plagioclase from the melt. At specified intense parameters (T, P, ZH2O) by the Pl composition, the model allows one to calculate Ca# of the melt and vice versa. In addition, the water content in the melt can be estimated from the co-existing melt-plagioclase. This exchange equilibrium does not allow one to estimate with a sufficient accuracy the temperature and pressure. Other models based on equations of crystallization equilibrium (for example, Ariskin and Barmina, 1990) can be used to estimate the crystallization temperature of plagioclase.
The convergence of the calculated and experimental data is presented in Fig. 1.
Fig.1. Convergence of calculated (equations 1-5) and experimental data.
To check calibration of the exchange reaction, Fig. 1a was supplemented with additional data (Bindeman et al., 1998), which were not included in the data set according to which the model was calibrated. As can be seen in Fig. 1, these data agree well with the calculated data. Absolute errors for the calculation of Ca# (Ca/Ca+Na) of the melts are shown in Fig. 1b. It is well seen that a calculation error is constant for a wide spectrum of compositions of plagioclases.
Calculated lines of the plagioclase-melt equilibrium for P = 1 kbar and T = 1000oC in dry and water-saturated systems are shown in Fig. 2. The composition of plagioclase crystallizing from the water-saturated system is strongly enriched in the An component as compared to the dry system (to 40 numbers of Al at Ca# of the melt 0.4-0.6).
Fig. 2. Calculated lines of the plagioclase-melt equilibrium for P = 1 kbar and T = 1000oC in the dry and water-saturated systems.
The calculated diagram for P = 1 kbar and natural co-existing plagioclase-melt pairs are compared in Fig.3. The data of Danyushevsky and co-authors (Danyushevsky et al., 1997) represents homogenized melt inclusions in plagioclase of high-calcium boninites of the Tonga channel. Temperatures (1040-1080oC), H2O content (close to water-saturated ones: ~1.5%), and crystallization pressures (0.25 kbar) were determined for them.
Fig. 3. Natural data on melt inclusions in plagioclase on the calculated (equations 1-5) diagram.
8
The data of Danyushevsky are arranged in the diagram along the isotherm of 1000oC and agree well with the model discussed. The data of Pletchov and co-authors (Pletchov et al., 1998) reflects the compositions of naturally quenched melt inclusions in plagioclase from basalts of the Klyuchevskoy volcano. The lowest-calcium group of the points represents co-existing interstitial glass-microlyte of plagioclase in the groundmass.
The differential trend of basalts with a gradual decrease in Ca# of the melt and An of plagioclase as the temperature decreases is well seen in diagram 3.
The model calculated makes it possible to estimate the plagioclase-melt equilibrium under dry and water-saturated conditions and to calculate the calcium content in the melt equilibrium to the specified plagioclase. This is necessary, for example, in studying melt inclusions in plagioclases when their equilibrium state with the mineral-host is estimated.
References :
#Chevychelov V.Yu., Zaraisky G.P., Borisovsky S.Yu. (IGEM, RAS). Solubility of columbite-tantalite in the melts of Li-F granite, and pegmatite-aplite banded rocks from the Orlovka tantalum deposit in Eastern Transbaikalia.
key words [granitic melts tantalum ore solubility ]
Tantalum ores from the Orlovka deposit assigned to albitite deposits (Smirnov et al., 1981) are localized in the apical part of the cupola of specific Li-F amazonitic granites, often enriched in albite. Presence of amazonite and specific banded granitic rocks composed of albitite (albite-rich), amazonite (microcline)-rich, and gresen-like layers with aplite or pegmatite texture are typical for this deposit (Zaraisky et al., 1997). The layered banded rocks form, as a rule, subhorizontal layers or flat lenses in the massive rare-metal granites. Their thickness varies from 10 cm to 1-1.5 m and the extension is up to several tens of meters. The joint spatial location of Ta ores and banded rocks may suggest the generality of physico-chemical and genetic conditions of their formation.
It appears interesting to estimate the possibility of tantalum to concentrate in individual layers of banded rocks, specifically those enriched in sodium. As geochemical observations and experiments (Alexandrov et al., 1985) show that tantalum-niobium mineralization is confined to albite-rich rocks, we believe that the banded rocks formation could proceed during crystallization of a volatiles-rich residual highly-evolved magmatic melt (Zaraisky et al., 1997). We, therefore, experimentally studied the solubility of columbite-tantalite (one of the basic ore minerals) in melts of the individual layers of banded rocks and massive Li-F granite. The compositions of the same granites demonstrate increased concentrations of F. As suggested by the experimental data of Manning (1981) the composition of the Li-F granite specimen (A-92), most enriched in albite, corresponds to the cotectic minimum for a granite melt contained ~3% F.
We took for our experiments each one specimen most typical of four rock types: massive Li-F granite, albite-rich, amazonite-rich, and greisen-like layers. Li- and F- rich homogeneous glasses were produced from those specimens. To that end we ground the specimen to powder, added it ~0.6 wt% LiF powder and ~5% 0.2 m HF solution and placed the obtained stuff into a Pt capsule. The melting runs were conducted in an internally heated gas pressure vessel at T= 1050oC, P= 1kbar for ~1 day. The glass was then taken out, reground and powdered in a mortar. The glass powder was again packed into a Pt capsule and the melting run was conducted under the same conditions for one more day. As suggested by the X-ray powder diffraction and microprobe analyses, the produced glasses were completely amorphous phases, quite homogeneous in the chemical composition. The glasses contained no less than 1 wt% F and ~2.5-4 wt% H2O.
Crystals of natural columbite-tantalite (in wt%: 55 Nb2O5, 20 Ta2O5, 13 MnO, 4.5 FeO, 2 SnO2, 1.3 WO3, 3.5 TiO2) were used in the solubility runs. A columbite crystal
# This work was supported by the RFBR (grant 96-05-64709) and RFBR-DFG (grant 96-05-00020G).
9
was placed into the glass powder with the addition of 0.2m HF solution. The runs were conducted in Tuttle bomb with water as pressure medium at T=820oC, P=1kbar, the duration of the runs was 15-25 days.
The mean chemical composition of the glasses after the solubility runs at ~15 microns away from the boudary with the columbite-tantalite crystal are listed in the table (microprobe analysis data).
Li-F granite (A-92) glass (gr) |
albite-rich glass (ab) |
amazonite-rich glass (kf) |
greisen-like glass (gs) |
|
SiO2 |
73.9 |
73.7 |
74.2 |
81.6 |
TiO2 |
0** |
0.1** |
0.1** |
0.1** |
Al2O3 |
16.0 |
17.3 |
15.4 |
12.6 |
FeO* |
0.3 |
0.4 |
0.1** |
0.2 |
CaO |
0** |
0.3 |
0.1 |
0.2 |
Na2O |
4.4 |
6.2 |
3.2 |
2.4 |
K2O |
4.4 |
1.3 |
6.2 |
2.2 |
Mn |
0.13 |
0.09 |
0.08 |
0.145 |
Ta |
0.18 |
0.125 |
0.14 |
0.10 |
Nb |
0.16 |
0.08 |
na |
na |
molar. Al/(Na+ +K+2Ca) |
1.30 |
1.34 |
1.23 |
1.76 |
*- Total iron as FeO; **- less than 2s standard deviation.
Mean concentrations of tantalum in the melts were from 0.1 to 0.2 wt%, of Mn from 0.08 to 0.15 wt%. These concentrations are 2-4 times as high as the initial ones.
Fig.1 exemplifies the concentration profile of tantalum resulting from dissolution (diffusion) of columbite-tantalite into a Li-F granitic melt. For this profile the concentrations of tantalum were analysed in 15 points away by 10, 15, 20, and so on to 400 microns from the columbite crystal boundary. The approximation of the analyses using the equation y=0.08+3.9/(x+27) yields ~0.22 wt% Ta in the melt at the boundary with columbite.
Fig.2 gives the concentrations of tantalum and manganese measured simultaneously in each point using 2 crystal-diffraction microprobe spectrometers. The maximum concentrations of tantalum were obtained in the granitic melt, the minimum in the greisen-like one, and in the albite- and amazonite-rich melts the tantalum concentrations had intermediate values. The granitic and greisen-like melts are, comparatively, manganese-rich whereas the albite- and amazonite-rich melts are comparatively manganese depleted. The direct proportionality in the concentrations of tantalum and manganese is well seen for all the melts but the albititic one.
Fig.3 illustrates the dependence of the mean concentrations of tantalum on the value of the molar Al/(Na+K+2Ca) ratio in the melt. The dependence is inversely proportional. The analogous inversely proportional dependence is found between mean concentrations of tantalum and calcium in the melt.
Fig.1. Example of the concentration profile of tantalum resulting from dissolution of columbite-tantalite crystal into a Li-F granitic melt
Fig.2. Dependence of the tantalum contents on manganese in the melts of Li-F granite (gr), albite-rich (ab), amazonite-rich (kf), and greisen-like (gs) compositions
Fig.3. The effect of melt composition on mean contents of tantalum in the melts of massive Li-F granite and the individual layers of banded granitic rocks (abbreviations see Fig.2) at 820oC and 1 kbar
The solubility values obtained by us from natural columbite-tantalite are quite comparable with the data of Linnen and Keppler (1997) who studied the solubility of synthetic columbite (MnNb2O6) and tantalite (MnTa2O6) in alkalic synthetic haplogranitic melts of various compositions, although the melt compositions studied by us had higher concentrations of alumina and a higher value of the Al/(Na+K) ratio. In accord with the experimental results of Linnen and Keppler (1997) this solubility ought to dramatically increase with alkali contents in water-saturated peralkaline melts and their temperature.
Concluding statement
10
The solubility of columbite-tantalite in a Li-F granitic melt, determined by us, has a noticeably lower value as compared with peralkaline granites. The experimental results show that the concentration of tantalum in the melts of individual layers of banded rocks from the Orlovka deposit is lower than in a non-layered Li-F granitic melt. It seems, therefore, highly unlikely that the tantalum mineralization is genetically related with the formation of banded granitic rocks bodies.
References:
Kotova A.A., Fed'kin M.V., and Osadchii E.G. Measurement of fO2 in five ordinary chondrites and redox conditions in the parent bodies of meteorites.
key words [oxygen fugacity chondrites]
Oxygen fugacities (fO2) were measured in ordinary chondrites (Okhansk (H4), Savchenskoe (LL4), Elenovka (L5), Vengerovo (H5), and Zhigailovka (LL6)) by the high-temperature emf-cell technique. The results are presented in the form of the following equations:
lgfO2(Okhansk) = -31256.4/T(K) + 10.7 (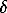 =+0.97; 1070<T(K)<1270)
=+0.97; 1070<T(K)<1270)
lgfO2(Vengerovo) = -28722.8/T(K) +8.2 (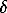 =+0.99; 1050<T(K)<1230)
=+0.99; 1050<T(K)<1230)
lgfO2(Elenovka) =-30068.0/T(K) + 9.7 (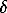 =+0.98; 1050<T(K)<1230)
=+0.98; 1050<T(K)<1230)
lgfO2. (Savchenskoe) = -32903.1/T(K) +12.7 (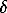 =+0.97; 1070<T(K)<1270)
=+0.97; 1070<T(K)<1270)
lgfO2(Zhigailovka ) = -29311.1/T(K) + 9.153 (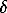 =+0.98; 1050<T(K)<1230)
=+0.98; 1050<T(K)<1230)
The accuracy of the fO2 measurements was +0.021logfO2.
The data obtained suggest that fO2 increases from H-chondrites to LL-chondrites and decreases with increasing degree of metamorphism of chondrites within each chemical group.
All the chemical groups of ordinary chondrites have similar phase composition. The main phases are olivine solid solution ((Mg,Fe)2SiO4), orthopyroxene (Mg,Fe)SiO3, kamacite ( Fe-Ni), taenite (
Fe-Ni), taenite (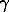 Fe-Ni), and troilite (FeS). All the groups of ordinary chondrites differ in composition of individual phases, in particular, in FeO content in the olivine and pyroxene solid solutions and the Fe/Ni ratio in the taenite and kamacite solid solutions. These variations are responsible for the difference in the equilibrium fO2 values determined for different chemical groups of ordinary chondrites at a constant temperature.
Fe-Ni), and troilite (FeS). All the groups of ordinary chondrites differ in composition of individual phases, in particular, in FeO content in the olivine and pyroxene solid solutions and the Fe/Ni ratio in the taenite and kamacite solid solutions. These variations are responsible for the difference in the equilibrium fO2 values determined for different chemical groups of ordinary chondrites at a constant temperature.
Thus, at relatively high temperatures (1000-1300 K), all originally nonequilibrium phases of ordinary chondrites come to equilibrium. For each equilibrated meteorite sample, fO2 (under closed system conditions) is a function of meteorite composition and temperature only at constant phase composition. In this case, the oxygen fugacity can be used as a criterion for quantitative classification of chondrites belonging to different chemical and petrological types.
References :
#Suk N.I. Experimental study of silicate-carbonate immiscibility of alkaline melts.
key words [silicate-carbonate immiscibility experiment]
Carbonate-silicate layering of melts at T = 1100oC and PCO2 = 2 kbar was experimentally studied in agpaite and lime systems, which can model the separation of carbonatite melts from alkaline agpaite and lime magmas. The experiments were carried out in alkaline and lime systems (albite-carbonate (Na2CO3 or Na2CO3 + CaCO3) and albite-diopside-carbonate) in sealed platinum capsules in the high-pressure gas apparatus for 6 h followed by quenching.
# This work was financially supported by the Russian Foundation for Basic Research (Project No. 97-05-64158)
11
A wide region of layering of the starting melts to two liquids (silicate and carbonate) was revealed. The silicate phase is a homogeneous glass in which the salt phase forms fine droplets and larger separations with a distinct phase boundary between the melts. The carbonate phases obtained were established to be heterogeneous, which is due to immiscibility of alkaline and lime carbonate melts (Figure). In the alkaline systems, the carbonate liquid is layered to the alkaline (predominantly sodium, up to almost neat Na2CO3) and alkaline-lime (predominantly calcium) fractions, whereas in the lime systems, the alkaline-lime fraction and almost neat calcium carbonate are separated.
The distribution of ore elements (REE (La, Ce), Nb, and Ta) between the melted phases was experimentally studied. It is established that in alkaline systems, rare-earth elements are concentrated in carbonatite melts enriched in calcium, whereas sodium phases contain almost no lanthanum and cerium (Fig. 1). By contrast, in the lime silicate-carbonate systems, rare-earth elements are concentrated in silicate melts, which are also rich in calcium. The results obtained allow one to understand the observed relationship between the rare-earth carbonatite deposits with alkaline agpaite magmatism. The preliminary results on studying the distribution of Nb and Ta between silicate and carbonate phases in alkaline experimental systems show the concentration of these elements in silicate melts.
In the magmatism development, lime-alkaline carbonate magmas retain the primary composition only in the volcanic surrounding (for example, carbonatite magmas of the Oldonio Lengai volcano in Tanzania, whose compositions are well comparable with the experimental data obtained by us for the alkaline systems). The experimentally revealed alkaline-lime separation of carbonate melts played most likely a certain role in the formation of carbonatites in intrusive ijolite-urtite complexes in which they are presented by calcite and dolomite types. It is likely that during their formation carbonates migrated with fluids into host rocks, which underwent alkaline metasomatosis (fenitization). The direct separation of lime carbonatites from silicate melts without agpaite specificity takes place in the case of kimberlite magmatism and is modeled by the experiments performed in the lime systems depleted in alkali metals. These experiments reveal instability of silicate melts with calcium excess from which calcite and dolomite carbonatites are separated.
Aleksandrov S.M. Endogenous borates in eruptive rocks imitating compositions of magnesial skarns.
key words [endogenous borates igneous rocks]
Endogenous borates being very abundant in magnesial skarns are also found in magmatic rocks. They are presented by minerals of the ludwigite-vonsenite series, chromoiron-containing variations of warwickite and kotoite, and in miarols of desilicylated pegmatites, they are presented by tusionite. Their compositions and genesis are studied.
In cavities of lavas and tuffs (trachytic in compositions) of Italian volcanos (Cimino, Osa, Lagetto, Libano, Tuoro, Vesuvius, island Volcano, and others) containing about 60% SiO2, 15-20% Al2O3, and less than 5% MgO, vonsenite [1,2] is known, whose composition varies from
Mg0.16Fe2+0.84)2(Fe3+0.94Al0.05)Fe2+ + Ti4+)BO3O2 to Mg0.015Fe2+0.985)2(Fe3+0.090Al0.10)BO3O2.
It is the product of condensation of volcanic fluids and concentrator of boron and maximally reduced iron, which were not required during magma crystallization.
The composition of trachytes is close to that of near-skarn rocks and rocks deposited on magnesial lime skarns in which vonsenite is an abundant borate. The melting temperature of vonsenite is its stability limit [2,3].
Another example of borate development is their substitution of the association of spinelides with variable composition and forsterite in dunites intruded by granatoids and gabbro ( in nickel deposits in Jumbo Mountain, Washington, USA, in the Hayama mine, Fukusima prefecture, Japan, and in chromite-containing dunites of the Voykaro-Synninsky massive, North Ural). Near-contact dunites contain vein-disseminated ludwigite.
These endogenous borates have variable contents of Cr and Al and small amounts of Co and Ni, which is their typomorphic specific feature. These elements, except Al, are absent in spinel-forsterite skarns with ludwigite in dolomite contacts, whose composition is closest to substituted ultrabasic rocks. The development of kotoite, Mg3[BO3]2, in dunites on Jumbo Mountain is also confirmed [4,5].
According to our data, the composition of ludwigite in this deposit varies from
(Mg0.73Fe2+0.27)2(Fe3+0.98Al0.02)BO3O2 (at Cr2O3=0.02% and CoO = 0.1%) to
(Mg0.78Fe2+0.22)2(Fe3+0.67Cr3+0.16Al0.17)BO3O2 (at Cr2O3 = 6.04%, CoO = 0.04%). Magnesioludwigite from Japan studied by us has the following composition: (Mg0.975Fe2+0.025)2(Fe2+0.025)2(Fe3+0.83Cr3+0.07Al0.10)BO3O3 and contains Cr2O3 = 2.87%, NiO = 0.13, and CoO = 0.09% [5].
Warwickites in dykes of forsterite-containing lamproites (jumellites) and carbonatites in Murcia province (Spain) are another type of endogenous borates of intrusive rocks [5, 6]. In accessory warwickites, whose composition in jumellites varies from Mg[Fe3+0.63Cr3+0.02(Ti4+ +
12
+ Mg)6+0.34]BO3O to Mg[Fe3+0.75Cr3+0.02(Ti4+ + Mg)0.23BO3O with the content of Cr2O3 from 0.78 to 0.81%, 0.n% Zn and Nb are present. In carbonatites, the composition of warwickite varies from Mg[Fe3+0.43Cr3+0.18Al0.01(Ti4+ + Mg)0.38]BO3O to Mg[Fe3+0.95Cr3+0.01(Ti4+ + Mg)0.04]BO3O against the background of Cr2O3 from 9.64 to 0.67% and TiO2 from 10.15 to 0.79 wt.%. The extent of heterovalence substitution (Ti4+ + Mg)6+ 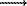 2Fe3+ in borates of magmatic rocks is higher than 50 mol.%, whereas in magnesial skarns, it is less than 50 mol.%.
2Fe3+ in borates of magmatic rocks is higher than 50 mol.%, whereas in magnesial skarns, it is less than 50 mol.%.
The comparison of compositions of lamproites with spinel-pyroxene-forsterite skarns and those of carbonatites with spinel-humite-forsterite calciphyres indicates that the intrusive rocks of Murcia province imitate contact metasomatites with respect to dolomites [7]. The composition of borates in them is predetermined by magnesiality of forsterite, the type of Cr-Al-Fe-Ti-spinelides in them, and existence of Zr and Nb characteristic of the types of intrusive rocks under discussion [5, 6].
The study of genesis of endogenous borates in intrusive rocks was predetermined by the previously established correlation of compositions of near-contact zones of magnesial skarns and accesory magmatic rocks with different petrochemical compositions [7]. It was shown that the borate mineralization is absent in skarns accompanying gabbro (SiO2 < 60%) and dunites, while in contacts with kimberlites, lamproites, and carbonatites (SiO2 << 40-36%), dolomites are only marbelized at the progressive stage of metasomatism. The logical assumption on the possibility of the appearance of borates as accessory minerals in these types of magnesium-containing rocks [4, 6], but unsaturated with silica, was confirmed by the data presented above.
References:
#Chashchukhin I.S., Votyakov S.L., Uimin S.G. and Mironov A.B. Redox state of Alpino-type ultramafites in formation of high-chromium chromite mineralization (by an example of the main ore field of Kempirsaiskii massif, Kazakhstan).
key words [redox state chromite mineralization]
The compositions of co-existing chrome-spinelides and olivines were studied in two types of chromite mineralization: in lean- and rare-disseminated chromitites of the Geofizicheskoe XII deposit (type I) and heavy-disseminated and continuous ores of the Almaz-Zhemchuzhina deposit (type II). Fifty samples were studied, the oxidation state of iron was determined by NGR analysis data for 22 of them. For chromitites of the I type, the situation characteristic of magmatogenic deposits was established: a regular growth with the depth of the mass fraction of chrome-spinelide (from 1.5 to 40%) correlating with a decrease in its ferruginosity from 45 to 35 and ferruginosity of olivine from 7.5 to 5% and a decrease in the concentration of manganese and an increase in the concentration of nickel in chrome-spinelide (Fig. 1). The inverse situation is observed for chromitites of the II type: ferruginosity of olivine and chrome-spinelide increases regularly from 4.4 to 9.0 and from 27 to 35%, respectively, with depth and a parallel increase in the content of Mn and a decrease in the content of Ni in chrome-spinelide (Fig. 2). Taking into account the data on the behavior of ore bodies in the geological structure of the South-Eastern block of the Kempirsaiskii massif, we can assert that chromitites of the II type have the metasomatic, lateral-segregation nature (Chashchukhin et al., 1997).
To elucidate the conditions of formation of the chromite mineralization by the oxygen thermobarometric method (Ballhaus, Berry, Green, 1991), we studied variations of redox state of chromitites of the both types by vertical. It is established that within ore bodies, the fO2 value is not constant and changes regularly, and the characters of these changes differ in different types of chromitites. For example, in the central part of the magmatogenic ore body (Fig. 1) and in the bottom part of the metasomatic body (Fig. 2), we detected the most reduced redox state corresponding in value to the mantle state. In other parts of the ore bodies, the oxygen fugacity increases gradually and tends in the limit to fO2 of host rocks.
Thus, it can be supposed that the both types of chromite mineralization were formed under sublithospheric conditions. Similarity (but not identity) of compositions of ore-forming chrome-spinelides and primary fO2 values in chromitites indicate to a single source of reduced fluids, which were a reason for several successive mantle processes: magmatic depletion progressing with depth resulted in the rough lamination of ultramafites, crystallization differentiation in the very bottom layers of the chrome-spinel-olivine melt, and finally, the formation of metasomatic mineralization in the upper part of the dunite-harzburgite series.
# This work was financially supported by the Russian Foundation for Basic Research (Project No. 98-05-6500, 96-05-64532)
13
Fig.1. Variations by vertical of the chemical composition of co-existing chrome-spinelides and olivines and oxygen fugacity in chromitites of lean- and rare-disseminated ores open in hole 681 of the Geofizicheskoe XII deposit.
Fig.2. Variations by vertical of the chemical composition of chrome-spinelide and oxygen fugacity in chromitites of continuous and heavy-disseminated ores open in hole 222 of the Almaz-Zhemchuzhina deposit.
#Durasova N.A., Belyaeva V.K., Kochnova L.N., Troneva M.A., Bannykh L.N. State of molybdenum in alumosilicate glasses.
key words [molybdenum valent state haplogranitic melt]
Molybdenum forms a large number of components where it occurs in different oxidation degrees. In natural minerals molybdenum is found as Mo+6, Mo+4 and rarely as Mo+5[1]. The state of molybdenum in magmatic systems is studied insufficiently. The available assumptions of several authors that molybdenum occurs in magmatic melts in tetravalent form are based either on the data on selective leaching of various forms of the element from granite rocks or on energetic characteristics of Mo4+ ion. The latter, together with the supposed specific features of
# The work has been supported in part by the RFBR, project N 58-05-64275.
14
the silicate melts structure suggested that molybdenum can occur as a sulfide MoS2 and oxygen compleses of the type MoO32- or MoO44- [2].
Ryabchikov et al. [3] reported an observation of MoO2 precipitation in granitic melts with fO2 Ni-NiO.
In order to study the state, the degree of oxidation of molybdenum in alumosilicate glasses- analogs of acidic rocks under physical-chemical parameters real for natural systems, and to estimate the migratability of the element at the interaction of magmatic phases with acidic solutions, we have carried out a sythesis of Mo-containing glasses of the granite eutectic composition at T=1200oC, fO2 of wustite stability region using a 'crucible-to-crucible' technique. The gate was corundum and graphite powders. Some samples in the form of grains were heated in air at a subsolidus temperature, then the initial and heated samples were washed with hydrochloric solutions. The experimental conditions are listed in the table.
Table. Synthesis conditions and concentration of molybdenum in HCl solutions (0.1 H) having contacted with granitic eutectic glasses.
N of sample and synthesis conditions |
Heating conditions of glass grains **** |
Glass charge, mg |
Solution volume |
Concentration of Mo in HCl solutions g /ml time of the interac. 30 min ***** |
Amounts of Mo escape from glasses, % |
31-Mo* T-1200oC t-1hr fO2-FeO |
Without heating, 500oC 10 days fO2 air |
|
|
|
|
32 Mo* T-1150oC t 1 hour fO2 FeO |
Without heating 400oC 10 days fO2 air |
51.0 50.0 |
5 5 |
0.25 0.42 |
1.3 2.6 |
33-Mo* * T-1150oC t-1 hour fO2 FeO |
Without heating 400oC 10 days fO2 air |
51.0 49.5 |
5 5 |
0.07 0.20 |
2.3 6.7 |
34-Mo* * * T-1150oC t- 1 hour fO2 FeO |
Without heating 400oC 10 days fO2 air |
50.9 50.8 |
5 5 |
0.71 0.93 |
2.3 3.1 |
* concentration of 'MoO2' in the charge - 0.2% ;** concentration of 'MoO2' in the charge - 0.04%; *** concentration of 'MoO2' in the charge - 0.4%;**** grain size 0.2-0.5 mm;***** atomic absorption determination in a cuvette , determination inaccuracy ~3-7%, sensitivity 0.001 g/ml.
The X-ray spectral analysis of the Mo-containing glasses was performed on a Camebax-Microbeam at an accelerating voltage 15kV, current 30nA, electron probe scanning being 20.20 mh.
The analysis lines were NaKa, SiKa, AlKa, MoLa, KKa. The observation limit of Mo in the glass on a crystal PET is about 0.1 wt % for the MoLa line (at a confidential probability 0.95).
Fig.1. ESR absorption spectra of isoelectronic Mo3+ and Mo5+ at 77 K in aluminum-zinc phosphate glass doped with 0.5 wt% MoO3 [4].
The glasses demonstrated microinhomogeneities, the occurrence of precipitates measuring about 5 microns which contained to 70% Al and to 0.9% 'MoO2'. The precipitates constituted to about 10% of the total area. The state of molybdenum in the glasses was studied by the ESR. The paramagnetic ions detectable by the ESR are Mo5+and Mo3+. We studied the initial and heated in air varieties with molybdenum contents of 0.04; 0.2; 0.4 wt% 'MoO2'. The spectra were recorded on a Varian-4502-15a spectrometer at 295 and 77 K. The spectral parameters are given on the plots. R.J. Landry [4] reports ESR Mo3+ and Mo5+ spectra observed at 77K in aluminium-zinc-phosphate glasses synthesized in nitrogen in the presence of carbon (fig.1). The spectrum of isolated Mo3+ ions is in the region of low fields (g ~ 5) and that of the exchange pairs in the region g~ 1.93 (fig.2). No Mo3+ was found in alumosilicate phases either in small field with g~ 5 or in medium ones with g~ 1.93 investigated by us at temperatures 77 and 295K. Isotropic Mo5+ spectrum with g=1.906, A=87× 10-4cm-1 was documented in all the phases (fig.2). The ESR Mo5+ signal parameters remain independent of Mo content in the samples (0.04-0.4%), but the intensity grows appreciably in this case both in the initial and heated varieties equally. The spectra are independent of the magnetic field orientation and the temperature (295 and 77K).
The amounts of molybdenum escape from glass grains upon treating them with HCl solutions have been established (table).
15
Fig.2.
The concentration of Mo in the solutions having interacted with the phases heated in air is greater than in solutions having contacted with the starting samples.
In our studies we were the first to establish the occurrence of molybdenum in the form of Mo5+ in granitic eutectic glasses ( both in the samples synthesized at fO2 of wustite stability region and in the varieties heated in air). The obtained spectral characteristics can be suggestive of the fact that Mo5+ ion forms its own oxygen environment which is a distorted octahedron. The occurrence of Mo4+ and Mo6+ is possible in glasses undergone alterations. As suggested by our ESR data, the concentration of Mo5+ persists in either case herewith. Washing with HCl does not lead to a change in the Mo5+ concentration in samples. The intensity remains constant within the accuracy of the measurements. The migration of Mo from the heated phases grows insignificantly which may be related to a possible change of the molybdenum state ( to Mo6+).
References:
#Ivanov B.A. Thermal model of the impact magmatic body formation in the Sudbury astrobleme.
key words [impact magmatic body sudbury]
Introduction. The Sudbury Igneous Complex (SIC) is proved to be a solidified volume of the impact melt generated by a hypervelocity asteroid impact 1.9 Ga ago. The reconstruction of the post-cratering tectonic deformations allows one to restore the initial geometry of the now observed uneroded part of SIC as a sheet of melt covering the depression of 60 km in diameter and 6 km in depth. The SIC thickness is around 2.5 to 3 km, overburden with approximately 3 km impact-related breccia and postcrater deposits. The SIC body differentiated into 2 main layers: upper granofires and near-bottom norites are the main facies. The aim of the present work is to create a thermal model of the SIC formation. The model includes (1) a numerical modelling of the impact creater formation, and (2) an estimate of the cooling history of the SIC melt. The model allows one to construct initial and boundary conditions for the following geochemical models of the SIC differentiation.
The impact cratering model. The numerical simulation of the cratering event was conducted to assess the temperature field at the Sudbury impact site just after the modification of the transient crater. We used the SALE 2D hydrocode [1] with some modifications described in [2]. The numerical modelling was done for a stony (granitic) body impacting upright the granite target at the impact velocity of 20 km.s-1. After several code runs, a cylindric projectile, 12.5 km in diameter and height (or an equivalent spherical projectile of 14 km) turned out to fit the estimated volume of the impact melt of 8000 km3 for the Sudbury structure [3]. The numerical simulations allow one to estimate the transient crater depth and radius to be 40 km. The maximum depth of the impact melt zone is around 30 to 35 km considerably less than the mantle depth of 45 km estimated for the Sudbury region. It means that there is no direct admixture of the mantle material into the initial volume of the impact melt. The model calculations of the transient crater collapse allow one to estimate the temperature field under the SIC body. The distortion of the initial thermal field includes both the shock heating of rocks and the geotherms uplift due to the crater modification. Shock heating and structural uplift give a 'neck' of hot rocks with a radius of 50 km. Beyond this radial distance, hot ejected material lies on relatively cold target rocks, yet the pre-impact temperature field of the target lithologies remains nearly unchanged.
The cooling history estimates. Simple estimates with an ID implicit numerical code were performed to evaluate the cooling history of the SIC body. The geometrical constraints of the model are three flat layers, i.e., (1) overburden material with a thickness of 2.5 km, resting on (2) a 2.5 km thick melted layer, which in turn is underlain (3)
# This has been supported by the RFBR (Grant N. 96-05-64167)
16
rocks of the crust, uplifted due to the transient cavity collapse. The melt thickness in some test variants was increased to 4 and 6 km. The surface boundary conditions of layer (1) are held constant at a temperature of 300K; the temperature within layer (1) ranges from 300K ('cold breccia') to 850K ('hot suevite'). Melt layer (2) has a conservatively estimated initial temperature of 1800 to 2000K. For layer (3) we used the temperature profile derived from the numerical simulations presented above. Thermal constants used in our calculations were the same as in the thermal modelling of the Manicouagan crater [4]. The model shows that the time needed for the initial temperature to decrease below the liquidus point (assumed to be at 1450K) is about 100 ka, and below the solidus point (assumed to be at 1270K), about 300ka.
For the most extreme initial SIC thickness of 6 km, the times, to around 1 Ma. The enormous thickness of the melt layer (SIC), compared to other terrestrial impact structures, and its prolonged cooling, explain why the initial homogeneous impact melt at Sudbury underwent differentiation [5].
Conclusions. The numerical modelling of the Sudbury impact body formation gives initial and boundary conditions for a further study of a possible geochemical evolution of SIC. The enormous initial melt volume (more than 8000 km3) and the long time of solidification (around 1 My) allow the SIC to have a post-impact history similar to 'normal' endogenic magmatic bodies. Some peculiarities of the Sudbury geology (such as so called Offset Dikes) may be a consequence of natural tectonic deformations during the long time before the final solidification of the SIC.
References:
#Kol'tsov A.B. Models of postmagmatic platinum-bearing associations formation in layered basite-hyperbasite intrusives.
key words [basite hyperbasite intrusives postmagmatic associations]
A numerical modelling has been performed for the processes of postmagmatic alterations manifested in Precambrian basite-ultrabasite complexes of the Olanga group in North Karelia. The genesis of the sulphide and platinum metal mineralization in complexes of this type (Bushveld, Stillwater) are still controversial, there are arguments in favour of both magmatic and hydrothermal genesis of mineralization. Geologopetrographic study of layered complexes has shown that the richest platinum metal mineralization in them is confined to two groups of altered rocks: 1) high-temperature ones with the typical paragenesis Ath+Tlc+Act+Hbl+Chl, formed on gabbro-norites and pyroxenites; 2) low-temperature ones with the typical paragenesis CzO + Chl + Scp +Act +Qtz +Ms +Bt formed on gabbro-anorthosites and gabbro-norites [1].
A method of sequential reactors [2] with a variable fluid/rock ratio (W/R) was used in the modeling. The following versions of the processes, realized as dictated by the fluid flux power and time period of its interaction with rocks have been considered:
1) diffusional bimetasomatic interaction of neighbouring grains of different minerals without attaining an equilibrium in the whole of the rock volume; 2) autometamorphism with the formation of a new equilibrium paragenesis at a constant bulk chemical composition; 3) metasomatosis in the open system with quite mobile components where the sense of the rocks transformation is determined as the result of combination of a fluid source of a particular composition and the mechanism of the concentrated fluid flux formation (convection, compression, decompression) [3]. An analysis of mineral equilibria enables one to determine T=650-720oC for group I, 350-450oC for group II at P=2.5-3 kbar.
The modelling has shown that at metamorphism and infiltration metasomatosis of gabbro-norites and gabbro-anorthosites under elevated temperature conditions the most characteristic is the formation of associations with cordierite, sometimes with corundum and, also, complete amphibolization of gabbro-norites (to 85-90 vol% rock). These features are not characteristic of group I rocks. Their mineral composition and zonal structure are reproduced well in the model of diffusional intergranular interaction. There arises a sequence of zones Opx-AthAct-Na-Amp-Pl. Herewith mineral W/R values at which the substitution of Opx begins are by 2-2.5 orders lower than in the case of Pl which complies with the greater thickness of the reaction rims around Opx observable in the nature. Characteristic is also the formation of Na-rich amphibole compositionally close to pargasite at the expense of plagioclase. The Opx-Pl interaction occurs due to higher m CaO, m MgO at the side of Opx and higher m Al2O3 at the side of Pl, however, their equalization throughout the rock bulk does not take place which is possible at lower values of the integral fluid flux.
Under low-temperature conditions at the formation of group II rocks brittle deformations of rocks are possible with the formation of the system of fluid conductors that favours the manifestation of the infiltration metasomatosis. As contrasted from fluid systems in more acid rocks (granitoids, metapellites), cooling results in basites in se-
# This has been supported by the RFBR (Grant N. 96-05-6482)
17
lective transfer of Ca with the formation of magnesial Tlc-Chl, Act-Chl metasomatites due to an increase of the Mg/Ca ratio in the fluid with the growing T. Such changes are not characteristic of this group. In decompression model columns the formation of the paragenesis Czo+Chl+Act+Pl+Qtz (plagioclase is regarded here as an analog of scapolite) typical for these rocks is observed. Noncontrast transformations of rocks even at relatively high W/R values are specific which is related to the weak pressure dependence of the Mg/Ca ratio in the fluid in equilibrium with basites.
So, the characteristic feature of postmagmatic processes in the studied basite-ultrabasite complexes are the manifestation of the diffusional interaction of minerals under the high-temperature conditions and hydrothermal-metasomatic conversion of rocks at lower temperatures under decompression.
References:
Kravchuk I.F., Malinin S.D., Senin V.G., Dernov-Pegarev V.F. Partitioning of molybdenum between the phases of the fluid-magmatic system.
key words [molybdenum granitic melt fluid]
The process of elemental fluid-melt interaction should be regarded as the initial stage of formation of magmatic ore solutions. Molybdenum is classed with elements originally related with a magmatic source, and its partitioning in magmatic melts-fluids equilibria has been the subject of numerous experimental studies.
As follows from the available publications, despite the large scope of investigations performed, the regularities of Mo behaviour in fluid-melt equilibria remain a mystery in many respects.
The main reasons for the observable scatter in the available published data are as follows:
The following optimal conditions are proposed for conducting the runs:
We have persued the following objectives in this work:
The investigations concerned with the influence of chlorides on the partitioning of Mo have been performed using NaCl solutions of high concentrations 30, 60, 80 wt% and, in one case, water-free NaCl. In studying the systems, the principle of attaining the equilibrium on the two sided was kept where possible: by introduction of Mo into the initial glass and into the chloride solution.
Technique. The runs were performed in sealed gold capsules (the length 5 mm, the wall thickness 0.1-0.2 mm) in a high pressure cell provided with an external heating and the system of internal cooling that enable a practically instantaneous quenching of samples. The capsules were horizontally arranged which promoted an increase of the melt fluid contact region and ensured a better homogeneity of the temperatural field in the system (as compared with the vertical arrangement).
The phase composition determination was performed on an X-ray spectral microanalyser CAMEBAX MICROBEAM with four crystal-diffraction spectrometers. To reduce the effects associated with the action of an electron probe on the sample the 25.25 mm scanning mode of the probe was used. The silicate analysis was carried out at an accelerating electron voltage of 15 kV and the probe current of 20 nA.
Conclusions. The partitioning of molybdenum between the fluid phases of the composition H2O-NaCl of various concentrations from 0 to 100% NaCl and natural and synthetic alumosilicates melts was studied at 800oC and pressures of 1.0, 1.5 and 2.0 kbar. In the largest number of the runs the equilibrium was attained by preliminarily introducing the element alternately into the system with glass and solution.
It is shown that molybdenum occurs in the fluid solely in the form of a water compound throughout the range of
18
variable NaCl concentrations. No noticeable effect of SiO2 and Al2O3 concentrations in melts on the partitioning of molybdenum is found.
The established values of the partitioning coefficients of Mo, KMo=CF/CL, where C is the concentration of Mo in the fluid and the melt, respectively, at 800oC and 1.5-2.0 kbar amount to: granite - H2O-0.9+0.2; granite 1m NaCl -1.4+1.1; albite 1m NaCl-8.9+3.0; eutectic Ab-Q2-1m NaCl 10.9+4.0. The inverse correlation is found for the dependence between KMo and CaO in alumosilicate melt.
#Krigman L.D., Senin V.G. Peculiarities of olivine composition in equilibrium with larnite-normative alkaline melts.
Vernadsky Institute of Geochemistry and Analytical Chemistry RAS
key words [experimental petrology alkali magmas olivine]
Strongly silica-undersaturated high-calcium alkaline magmas are important for understanding the genesis and evolution of melilite-bearing rocks. High concentrations of CaO and alkali oxides combined with low silica content (SiO2 < 40 wt. %) result in a low degree of polymerization of such melts and determines their unusual chemistry.
We investigated the crystallization of synthetic multi-component larnite-normative batch compositions at atmospheric pressure (see table). The first of two studied compositions (olivine melilitite) is similar (for except of volatile components) to homogenized melt micro-inclusions found in early cumulate minerals of Kugda intrusion (Majmecha-Kotuj province of Polar Siberia). The second composition (turjaite) corresponds to the bulk average composition of turjaite dykes of the same intrusion (Egorov, 1991).
The runs were carried out in a vertical electric furnace. The samples were assembled using container-free loop method. We have done two sets of experiments at different redox conditions: 1) in air and, 2) at fO2 that corresponds to that of QFM buffer.
Table. Compositions of starting mixtures
SiO2 |
TiO2 |
Al2O3 |
FeO |
MgO |
CaO |
Na2O |
K2O |
P2O5 |
|
1 |
39.97 |
5.26 |
4.13 |
7.93 |
12.53 |
24.11 |
3.61 |
1.88 |
0.59 |
2 |
40.57 |
2.34 |
10.16 |
9.63 |
10.23 |
22.25 |
2.86 |
1.54 |
0.49 |
Olivine equilibrated with the liquid in our runs occurs as slightly zoning crystals (the diffusions zone make 2-4 mikrons, fig.1).
It is enriched in forsterite component despite rather high iron contents in coexisting liquids. Mg#ol for the olivine melilitite melts is close to 0.9. For turjaitic melts on the initial stages of their crystallization Mg#ol is slightly lower (~0.85) and decreases gradually down to 0.8-0.75 with increasing total iron content in the liquid. The constant of the exchange reaction Kd = (Feol/Mgol)(Fel/Mgl) for the olivine melilitite liquid equals 0.1-0.15 (we used the common iron as Fel for Kd calculation) and it has slightly higher value for the turjaitic melt (0.2-0.25). This is remarkably different from the values observed in basaltic systems (Kd = 0.3). We should note that in order to have Kd = 0.3 the proportion of Fe3+ should be 40-60 % for olivine melilitite melt and 30-35 % for turjaitic melt at fO2 = QFM, while in basaltic melts at the same conditions the proportion of Fe3+ is close to 0.1-0.2. This difference in oxidation state of iron seems to reflect the well-known effect of peralkalinity on the activity of ferric iron (Nikolaev et al, 1996).
Fig.1. Diffusion profiles for Fe, Mg, Ca and W across olivine crystal with microinclusion of melt. Olivine melilitite modified 8% WO3, 1170 oC, 3 hours, fO2=QFM. Step of scanning is 2 microns. The elements concentrations of melt and inclusion are approached. Apparent positive peaks in Ca and W concentrations are result of scheelite nucleation
Fig.2. CaOolivine versus CaOmelts on larnit-normative melts compared with melilite nephelinite and alkalic basalts. Symbols: rhombuses - olivine melilitite; squares - turjaite; triangles -melilite nephelinites (Gee&Sack, 1988); crosses - ankaramite of Gran Canary (unpublished data of authors); circles - alkalic lavas (Gee et. al., 1987).
# We aknowledge support from INTAS-RFBR 19 Another striking feature of olivine composition is very high Ca content. Concentrations of CaO in olivine do not change much with variations of CaO in coexisting melts and reach 1.5-2 wt. % in turjaite composition and 2-2.5 in olivine melilitite system (fig.2). The values of equilibrium exchange constant Kd = (Caol/Mgol)/(Cal/Mgl) are 8-10 times lower than those of the constant for iron. Olivine with similar CaO concentrations was found in association with monticellite in the rocks of Maimecha-Kotui province of Polar Siberia (Egorov, 1992). High CaO contents were also detected in the rims of olivine phenocrysts and in small not zoned ground-mass laths of Zeughaus olivine melilitite dyke (Seifert and Thomas, 1995). The both volatile-free compositions of these rocks is close to that olivine melilite used in our experiments. It is interesting to note that the zoned olivine phenocrysts contain monticellite inclusions. In most cases CaO concentrations in olivines from melilite-bearing rocks are 0.2-0.5 wt. %. This implies that olivine found in the rocks may have crystallized from a melt of different composition, or that Ca may be lost by subsolidus exsolution. Monticellite inclusions in olivine described by Seifert and Thomas (1995) is a circumstantial evidence that exsolution may take place at subsolidus temperatures. References: #Lebedev E.B., Kadik A.A., Kuskov O.L., Dorfman A.M., Lukanin O.L. Experimental study of sulphide phases accumulation mechanisms with regard of ratio: basic melt - olivine (simulation with use of a high temperature centrifuge). key words [sulphide iron olivine accumalation centrifuge] Vernadsky Institute of Geochemistry and Analytical Chemistry, RAS, Moscow, Russia, elkor@geokhi.msk.su) An occurrence of large scale Earth's material melting processes is assumed at the early stages of Earth formation. This melting comprised the carbon-containing and sulphide-containing rocks which composed the high horizonts of the mantle. The metal and sulphide phases occurrence is assumed for this rocks. The determination of possible conditions for the metal and sulphide phases segregation from partial melting zones, its accumulation and movement to the forming Earth's and Moon's cores are the important problems. The experimental goal was a determination of the mechanisms of metal, sulphide and silicate phase differentiation in gravity field. Sulphide and metal phases have a particularity in its sursace energy, which differs them from the silicates when this phases accumulate in the inner Earth. Sulphur presence leads to the metals melting temperature decrease and development of silicate crystalline phases surfaces wetting by metal melts. Sulphide and ferrous-sulphide eutectic melts with the presence of basic silicate melt were used in the experiments. Experiments temperature was 1200-1400oC. The centrifuge ensured the acceleration of 3000-5000 g. The duration of centrifugation was 15 minutes. Centrifugation was carried out on mixture of iron sulphide FeS and silicate matrix composed of different proportions of olivine crystals and basic melt (basalt). The initial mixture were: olivine crystals from 50 to 90% and basic melt (basalt) from 50% to 6%. The initial amount of iron was 10% from silicate part. After centrifugation in the samples 3 following zones usually appeared. 1 - emerged basic melt zone; 2 - a zone containing mainly olivine crystalls (crystal matrix); 3 - a zone containing accumulated and sinked metalic and sulphide phases. In the three series of experiments carried out sulphide and metal phases accumulation was in the following initial proportions. 1 - olivine crystals (45%) and basic melt (basalt) 45%. 2 - olivine crystals (67.5%) and basic melt (basalt) 22.5%. Sulphide iron amount was 10% of silicate part. At the same time sulphide and metal phases accumulation has not occurred at the initial proportions: olivine crystals (8.45%) and basic melt (basalt) 5.5%. Sulphide iron amount also was 10% of silicate part. So, in the cases of mixture of olivine crystals, basic melt and sulphide melt (FeS) using a liquid metal penetration through the crystal matrix containing olivine (from 50 to 80 %) and basic melt (basalt) (from 50 to 10 %) was an evidence. Probably, the 6% -content of basalt melt in olivine matrix is a value close to minimum silicate liquid concentration permitting sulphide iron penetration through silicate matrix and its accumulation in a separate phase. It is possible to assume that such proportion of basic melt, olivine crystals and sulphide iron is enough for metallic sulphide phase segregation and accumulation. On the basis of the data obtained it is possible to assume that sulphide and metal phases segregation and accumulation in the silicate matrix analogue of mantle rocks can occur in the case of partial melt concentration equal or higher than 6%. Lukanin O.A. The behaviour of Cl and H2O at degassing of rhyolitic and pantelleritic magmas (numerical modelling results). # This is supported by the RFBR (Grant N. 98-05-64768) 20 key words [degassing rhyolite pantellerite melt Cl] GEOKHI The propensity of Cl to form stable chloride-metal complexes with many basic, rare, and ore elements in the water-containing fluid phase plays very important role in extraction of these elements from magmas and their transport by high-temperature fluids. As suggested by experimental data, at an acidic silicate melts-water fluid equilibrium Cl partitions preferentially to the fluid phase. The concentration of Cl in water-containing melts and the partitioning coefficient of chlorine between a fluid and a melt, DCl, depends both on the TP conditions and, largely, on the melt composition. Under the given PTX conditions (800-1000oC, P<2 kbar) due to higher (Na+K)/Al ratios and higher FeO* concentrations the solubility of Cl in a pantellerite melt is 2 to 3 times as high as in a rhyolite melt. It is worth noting that DCl depends significantly not only on the concentration of basic petrogenic melt components but, also, on the concentration of Cl in a silicate melt (Shironosova et al., 1989; Malinin et al., 1989; Webster, 1992; Webster 1997 et al). See, also, in reviews by Carroll, Webster, 1994; Malinin, Kravchuk, 1995. This report gives the results of a numerical modelling of the behaviour of Cl and H2O upon degassing of water-containing acidic magmas of rhyolitic and pantelleritic composition. Various versions of decompression- and crystallization-induced degassing of melts were calculated using computer models based on the available experimental data both under closed conditions where the fluid phase remains in the system and under the open conditions where the segregating fluid phase escapes form the system. For all the versions the initial degassing conditions were preset by the parameters at which the segregating chlorine-containing water fluid phase was homogeneous and the water-chloride melt was absent. So, the initial rhyolite melts containing <0.3 wt% Cl and pantellerite melts containing <0.6 wt% Cl were considered. At the decompression-induced degassing the concentrations of Cl and H2O in the melt and the fluid phase were considered with any one preset step of decompression in the system. The modelling of the crystallization- induced degassing was performed for isobaric conditions with a preset step of the initial melt crystallization degree increment. In this case we considered a simplified case of 'eutectic' crystallization where the temperature and the composition of the residual melt remained constant with respect to petrogenic elements. The obtained computated data suggest the following conclusions. 1. The fluid segregation from the melt in the process of the acidic magmas uplift to the surface and, accordingly, upon decompression, leads to a decrease of the Cl concentration in the melt. The degree of the Cl extraction from the melt at the surface is herewith the greater the higher are the initial concentrations of both H2O and Cl in the melt. The temperature decrease and the magma degassing under the open conditions with the escape of the segregated fluid phase from the magma increase the loss of Cl (figs.1 and 2). Fig.1. Change in the Cl and H2O concentrations in the rhyolite melt upon the decompression-induced degassing under the closed and open conditions (shown in black circles). Fig.2. Change in the Cl and H2O concentrations in the pantellerite melt upon the decompression-induced degassing under the closed and open conditions at temperatures 1000 and 800oC. Black squares stand for the initial H2O and Cl concentrations in the melt. 21 2. The decompression-induced degassing under the closed conditions of even strongly water-enriched pantellerite and rhyolite melts (H2O=4-7 wt%) does not lead to a dramatic loss of Cl by the melt. So, for example, in the case of degassing of pantellite magmas with the initial concentrations of H2O=7wt% and Cl=0.5 wt% (800-1000oC) the Cl loss at the surface does not have to exceed 40-50% of its initial concentration. A relative loss of chlorine, at degassing under the closed conditions, from pantellerite melts with lower initial Cl concentrations ().1 0.2 wt%) has to be still smaller (<20-30%) (fig.2). 3. The crystallization induced degassing of acidic melts (P=const) with low and moderate initial H2O concentrations (rhyolite <2-2.5 wt%, pantellerite <3-5 wt% H2O) leads to a significant increase of the Cl concentration in the residual melt despite the fact that DCl (fluid. melt) >1. Interestingly, that in the open system the degree of the Cl concentration increase turns out still more considerable than in the closed system (fig.3). Herewith, both in the case of rhyolite and pantellerite the Cl concentrations in the melt and the fluid phase do not reach the values at which the fluid starts layering with the formation of the water-containing chloride melt phase. Note, that for pantellerite the effect of the Cl concentration increase in the melt upon the crystallization-induced degassing can take place only at the H2O concentrations which correspond to the pressure range characteristic of the occurrence of the near-surface magmatic chambers (~1-2 kbar). Fig.3. Change in the Cl concentration in the pantellerite melt upon the crystallization-induced degassing (T=800oC) as a function of the amount of the residual liquid. The trajectories are shown for the melts with different initial H2O concentrations. References: #Shchekina T.I., Gramenitsky E.N. Features of scandium partitioning between alumosilicate and alkalialuminofluorine melts at 800oC and 1000 bar. key words [scandium fluorine granitic melt] M.V.Lomonosov Moscow State University The principal form of occurrence of Sc in nature is dissemination in rock-forming minerals. Out of magmatic deposits the largest ones are ilmenite-titanomagnetite deposits in basic and ultrabasic rocks where Sc concentrates in ilmenite and clinopyroxene. But there are true scandium deposits confined to granitic pegmatites wherein scandium mineral, i.e. thortveitite, is abundant and, also, skarn and greisen ones where such minerals as micas, beryl, wolframite, and others are scandium rich. The largest number of scandium deposits of the greisen type demonstrate a close association of scandium-bearing wolframites, cassiterites, beryls with topaz and fluorite, that is they demonstrate an apparent participation of fluorine in the ore formation process. In this respect our experiments concerned with the behaviour of Sc in a fluorine-containing granitic system can clear the problem of the mechanism of Sc concentration in deposits genetically associated with granites. We have studied experimentally the partitioning of Sc between silicate (L1) and alkalialuminofluorine (L2) melts which from in a fluorine-containing granitic system at 800oC and PH2O=1000 bar as a consequence of immiscibility at fluorine concentrations in excess of 3 wt%. It turned out that Sc fractionates contrastingly between two melts and possesses a pronounced capability of concentrating in salt fluorine melts of Me3AlF6 stoichiometry , where Me is Na, Na=K, or Na+K+Li. The quantitative data on partitioning of Sc between glasses, obtained as a result of melt quenching, have shown that in all parts of the system the partitioning coefficients (KD=CL1/CL2) are smaller than unity. They remain close, only slightly growing (from KD= 0.12 to 0.15) as the agpaitness coefficient of the system decreases and the concentration of # This has been supported by the research programm "Universities of Russia-basic Research" project 5013 22 potassium increases. The introduction of 1.3 - 2,5 wt% Li into the system leads to a greater than one order of magnitude concentration of Sc in alkalialuminofluorine melt and decrease of Sc content by a factor 2-3 in silicate one. In large globules formed by this melt (L2) in the Li-containing part of the system one can observe under an electron microscope small precipitates of light quenched phases of Sc fluorides. The transition from the quartz to the nepheline-normalized system's part leads to the equal partitioning of Sc between the two melts, and KD approaches 1. Of many other elements, W and Li appear most close to Sc in behaviour in all the compositions considered. Rare earth elements and yttrium which often associate with scandium in nature behave in an analogous manner in experiment only in the Li-containing part of the system concentrating, like scandium, in aluminofluorine melt. Similar partitioning in favour of the aluminofluorine melt is also found for Y and light rare earth's in purely Na system's part. In other cases their behaviour is different. The association of scandium-bearing and fluorine-containing minerals, observable in nature, is indicative of the fact that fluorine played an important role at the later magmatic stage and at the escape of Sc from a magmatic chamber. So, one can conjecture that Sc concentrating in a salt fluorine melt occurring in the form of droplets in the granitic melt interior could be a constituent of later minerals (micas for example) which crystallized from the melt. At the pegmatitic and hydrothermal stage Sc could from its own minerals and, also, concentrate in other Sc-bearing minerals coming into them from salt or silicate-salt melts which have a lower crystallization temperature as compared with a granitic one. #Shornikov S.I., Archakov I. Yu. and Shul'ts M.M. Partial coefficients of evaporation of silicon dioxide. key words [silicon dioxide evaporation] In this work, in terms of the mass spectrometric effusion Knudsen method, we studied evaporation of silicon dioxide samples from molybdenum chambers in the 1610-1980 K temperature range on a MI-1201 mass spectrometer modified for high-temperature studies. Effusion chambers were heated by a tubular tantalum heater, and the temperature of the chambers was determined by a WRe5/WRe20 thermocouple. The inner diameter of the effusion chambers was 8.00+0.05 mm, the height was 15.0+0.05 mm. The effusion chambers with diameters of holes equal to 0.308+0.007, 0.434+0.007, and 0.507+0.007 mm were used. Silicon dioxide samples were evaporated from the open surface (according to Langmuir) using a cylindrical effusion chamber without cover. To decrease fragmentation processes, the energy of ionizing electrons was 20 eV. The following molecular ions were observed in the mass spectra of vapor above silicon dioxide: (SiO)+, (SiO2)+, (Si2O2)+, (MoO)+, (MoO2)+, and (MoO3)+. The effective partial vapor pressures of (SiO), (SiO2), and (Si2O2) above [SiO2] were determined by the complete evaporation method using the Hertz-Knudsen equation. In the case of evaporation of [SiO2] from the cylindrical chamber, these partial pressures were determined by the comparison of ion currents using as the standard the partial pressures of silicon monoxide vapor obtained previously by the Hertz-Knudsen equation. The determined partial pressures of the molecular forms of (SiO), (SiO2), and (Si2O2) vapor corresponded within errors not greater than 15% to those calculated using partial pressures of silver vapor as the standard. The partial pressures of silver vapour were chosen, because evaporation coefficient of silver is equal to unity, which is the most important factor for studying evaporation processes from effusion chambers with different geometries. The values of partial pressures of silver vapours at temperatures of 1235-1450 K determined in this work are independent of the value of the diameter of the holes of the effusion and cylindrical chambers and coincide (within errors not greater than 5%) to those recommended in [1]. The value of the partial evaporation coefficient corresponding to the molecular form of vapour above the substance studied can be experimentally determined from the values of effective partial pressures of the given form of vapor obtained by evaporation of the substance from effusion chambers with different surface areas of effusion holes [2]. Another approach to experimental determination of partial evaporation coefficients is the comparison of values of partial pressures of vapour of molecules in the effusion chamber (according to Knudsen) and above the open surface of the studied substance (according to Langmuir) using the evaporation coefficient of silver in the liquid phase as the standard. However, the determination of value of partial evaporation coefficients corresponding to the components of the gas phase above silicon dioxide in terms of the Knudsen method using different geometric parameters of effusion chambers has considerable errors in the values determined. In the case of silicon dioxide, the approach of comparison of the data found by the Knudsen method to the information obtained by evaporation from the open surface results in overestimated values of partial evaporation coefficients of the components of the gas phase. The approach used in this work and based on the determination of equilibrium partial pressures of components of the gas phase from effective, experimentally determined values seems to be optimum. In this work, the equilibrium partial pressures of (SiO), (SiO2), and (Si2O2) vapours above silicon dioxide were determined by the least-squares method from the dependences of the partial pressures of vapour of these molecules on the f parameter characterizing the geometry of effusion chambers. The determined equilibrium partial pressures can be used for the determination of partial evaporation coefficients corresponding to the components of the gas phase both in terms of the Knudsen method on the basis of the Whitman-Motzfeldt [3, 4] and Komlev [5] equations and in combination of the Knudsen and Langmuir methods. This approach provides obtaining more reliable results as compared to the procedures already used. The use of the correlations based on the Komlev equation provides, in the general case, obtaining more correct results as compared to those following from the # This work was financially supported by the Russian Foundation for Basic Research, project no. 97-03-33414a 23 Whitman-Motzfeldt equation. However, in the case of evaporation of silicon dioxide, both approaches are equivalent. This is related to the fact that the values of partial coefficients of silicon dioxide are of an order of ~10-2 and far from the limiting cases. It follows from the data of the present work and published data that the partial evaporation coefficient corresponding to silicon monoxide at 1610-1980 K is constant and equal to (2.23+0.30).10-2. The estimates of the value of the integral evaporation coefficient of silicon dioxide obtained by the authors of [2] at 1692-1739 K do not contradict to that determined in [6] and corresponding to (SiO) in the temperature range from 1820 to 1960 K and the results of this work. The values of partial evaporation coefficients determined in this work and corresponding to (SiO2) and (Si2O2) molecules containing in the vapour above silicon dioxide are equal to (1.8+0.41).10-2 and (1.62+0.47).10-2, respectively. References : #Surkov N.V. and Gartvich Yu.G. Experimental study of phase interrelations in the enstatite-anortite facies in the CaO-MgO-Al2O3SiO2 system. key words [enstatite anortite melt experiment] The phase diagram of the CaO-MgO-Al2O3SiO2 system can be divided into two subsystems: forsterite-normative and quartz-normative, depending on the position of the specific region of compositions relative to the enstatite-wollastonite-corundum facies. The forsterite-normative region is studied in more detail than the quartz-normative region, which is due to a greater interest in it of petrologists and to a sufficiently evident analogy between the mineralogical set of plutonic rocks and associates of phases in the systems. Similar analogies are more complicated for the quartz-normative region of compositions. This is related to the considerable difference of the composition of quartz-containing plutonic rocks from the CaO-MgO-Al2O3SiO2 system, in particular, by the content of alkalis. Nevertheless, phase interrelations in the quartz-normative part of the CaO-MgO-Al2O3SiO2 system can serve as the simple model of quartz- and kyanite-containing parageneses of plutonic rocks and are the necessary basis for studying the influence of alkalis, the ferrous component, etc. on the stability of quartz-normative mineral associates. Therefore, we experimentally studied the enstatite-anortite facies within the pressure range of 12-15 kbar. The results are shown in Figs. 1-3. The experimental part of the work was carried out on a high-pressure apparatus of the "plunger-cylinder" type. The main specific features of the procedure have been described previously (Surkov, 1995; Surkov, Kuznetsov, 1996). The phases were diagnosed by the X-ray phase method and studying the petrographic slide, and the phase composition was determined on an electron microanalyzer. The position of curves of monovariant equilibria An+Opx = Gr+Cpx+Qz, Sil+An+Opx = Gr+Qz, and An+Opx = Gr+Cpx + L was studied. The results showed a good coincidence with the known data (Hensen, 1976; Hensen, 1981; Sekine, Wyllie, Baker, 1981; Perkins, 1983). The compositions of co-existing phases on liquidus and in solidus were established. The topology of nonvariant and monovariant equilibria in this region of compositions was analyzed. The position of nonvariant points (L, An, Opx, Gr, Cpx, Qz) and (L, Sil, An, Opx, Gr, Qz) was established. The diagram of the enstatite-anortite facies at 16 kbar was plotted from the results of the experimental study. In this facies, melting occurs according to the reaction An + Opx = Gr + Cpx + L in the 1375-1400oC range. The association of anortite and enstatite is replaced by associations of garnet with quartz, clinopyroxene, and kyanite in solidus as the temperature decreases. References : # This work was financially supported by the Russian Foundation for Basic Research, project no. 96-05-66036 24 Fig.1. Anortite-enstatite join at 16 kbar. Fig.2. Topological diagram of anortite-enstatite facies at 16 kbar. Fig.3. Topology of monovariant equilibria in the quartz-normative part of the CaO-MgO-Al2O3SiO2 system related to the nonvariant point (An, Opx, Gr, Cpx, Qz, Ky). 25 #Slutsky A.B. Pressure effect on the glass transition temperature (Tg) of anorthite glass. key words [glass transition temperature pressure]GEOKHI The glass transition temperature is an important parameter characterizing the properties and the structure of melt. At temperatures below Tg the temperature-dependent configuration reconstruction characteristic of the melt stops. Under normal pressures the glass transition temperature of silicate melts is derived from the calorimetric measurements data. Depolymerized melts SiO2, Na2O-SiO2 exhibit a most drastic jump-like change in the heat capacity upon the glass transition. For more complex melts the heat capacity jump magnitude decreases in the sequence: KAlSi3O8, NaAlSi3O8, CaAl2Si2O6, CaMgSi2O6. It is usually supposed that spectroscopic data on the quenched glasses structure can be used to understand the structure and properties of melts only in the case where these data were obtained at temperatures above Tg. As the data on the pressure dependence of the glass transition temperature are not available, we have carried out a search study in order to establish the possibilities of the glass transition temperature fixation by the method of electric conductivity measurement. Taking into account a usually good reproducibility of the measurements results and a high sensitivity of the electric conductivity to a structure modification, one could expect the appearance of a characteristic knee on the temperature vs the electric conductivity curve at Tg. The subject to study was a specially produced anorthite glass. We took anorthite glass precisely because should we succeed in fixing a change in the electric properties upon glassification of anorthite melt, this approach could be used to study the effect of pressure on the glass transition temperature of a number of other silicate melts. The study was carried out using the earlier designed technique for measuring the electric properties of solid dielectrics at high T,P. Taking into account the high resistivity of anorthite glass, we have modified the construction of the measuring cell and increased the sensitivity of the electric conductivity measurements at a frequency of 1 kHz for the purpose in question. Fig.1. Temperature dependence of anorthite glass electrical conductivity at 3 kbar. The electric conductivity measurements were performed in the range of 840-1240oC at fixed pressures of 3,6,9,12, and 15 kbar. Fig.1 gives the electric conductivity curve of anorthite glass in log The temperature vs the electric conductivity curves obtained at 6 and 9 kbar are analogous in form. The Fig.2. Pressure effect on the change of activation energy at Tg temperature. Fig.3. Pressure effect on the glass transition temperature of anorthite glass. Khisina N.P. , Wirth R.On the first finding of high pressure hydrous silicate inclusions in olivine. key words [olivine inclusion phase E] # This work has been supported by the RFBR (Grant N. 97-05-881, 97-05-390) 26 *V.I. Vernadsky Institute of Geochemistry, RAS 117975, Moscow. ** Centre of Geoscience (GFZ), Potsdam, Telegraphenberg. The present-day transmission electron microscopy (TEM), being a complex of local methods visualization, chemical and structural identification of the matter, is now finding its way into the province of micromineralogy where the volumes of the subjects under study are measured in nanometers and, therefore, beyond the reach of X-ray and microprobe techniques. The TEM combines the possibilities of spectral analysis of chemical composition and electron diffraction, enabling one to obtain simultaneously the chemical and structural information on the structure of mineral phases in extremely small microvolumes of the matter down to several nanometers. The traditional methods of electron microdiffraction (SAED), employing a parallel electron beam, make it possible to examine the matter with the locality of hundreds of nanometers. The use of CBED ( diffraction using a focused electron beam) increases the locality of the electron diffraction method making it possible to obtain a diffraction pattern of an area to 4nm in cross-section. This area can simultaneously be examined for the elemental composition by the method of the energy dispersion spectral analysis (ATEM) and, in a number of the cases using the electron energy loss spectroscopy (EELS) one can gain the information, with the same locality on the change in the valence state of the transition metals, and to carry out an analysis for the presence of light elements including (OH)- hydroxyl groups. The HRTEM (high resolution transmission electron microscopy) enables one to obtain a direct image of crystal lattice planes, and is indispensable for an investigation of various defects and nonstoichiometry of real crystals. Such complex of methods was employed to study the microstructure of olivine (Fa ~7.4) from a kimberlite mantle nodulus (the Udachnaya pipe) [1]. The preliminarily studied petrographic material [2] was kindly given to us by A.K.Ukhanov. An olivine grain, annealed at 700oC under laboratory conditions was prepared for the TEM in the form of an ion-thinned sample (~20 h m thick) oriented in parallel with a (100) plane. As suggested by the TEM, the olivine contained 50-500hm inclusions with the features of crystallographic faceting, having a strong moire contact. The area-averaged chemical composition of the inclusions corresponded to high ferrous olivine, however, a detailed ATEM analysis revealed a heterophase concentrically zoned structure of the inclusions with a layering in Fe, Mg, Si: the nucleus having the moire image contrast is Fe-rich and Mg-and Si-depleted; the rim has no moire contrast and stoichiometrically close (Mg:Fe:Si=62:5:33) to the olivine matrix whereas the olivine matrix at the border with the inclusions is drastically depleted in Mg and Fe and exhibits a high SiO2 concentration. The EELS spectra obtained separately from the nucleus and the rims of the inclusions had a 528 eV peak characteristic of the OH- groups [3] and Fe3+. The occurrence of this peak in the inclusions rims spectra with practically absent Fe in the rims indicated that the rim of the inclusions is the hydroxyl-containing phase; the occurrence of the 528 eV peak in the nucleus spectrum was indicative of the possible segregation of Fe3+. The concentrically zoned structure of the inclusions with the width of the zones comparable or smaller than that of the electron-microscope sample did not allow us to establish the compositions of individual phases and to identify the inclusions phases basig solely on the ATEM of a vertically inhomogeneous sample, despite the high area locality of the methods, a phase mixture is being analysed. The phases in the inclusions were identified by diffration methods SAED and CBED. The diffraction patterns were obtained with the electron beam orientation in parallel with [100], [311], [211] planes of olivine and were indexed as a superposition of hexagonal (trigonal) phase with the parameters a=2.93 A, c=13.8 A (phase E, rim); (iii) of the cubic (pseudocubic) phase with the unit cell parameter a=7.45 A (unknown Fe3+ - hydroxide, the central nucleus part); (iv) of secondary olivine (forsterite, nucleus). Strict dimension-orientation relationships have been found between the matrix and the inclusions phases. As suggested by the CBED data the inclusions rim is represented by the so-called phase E and the nucleus is represented by the preducts of oxidation and dehydration of phase E (Fe3+-hydroxide and secondary olivine) formed as a result of the laboratory annealing. Phase E is high-pressure hydroxyl-containing Mg, Fe-silicate having the formula Mg2.27Si1.26H2.4O6-Mg2.08Si1.16H3.20O6 [4] that was produced earlier only in high-pressure experiments and is regarded as a possible carrier of water in the Earth's mantle, but it has not been found in the nature until now. We believe that phase E inclusions found by us in olivine from the kimberlite mantle nodulus are a product of olivine hydration under the upper mantle high pressures (mantle hydrous metasomatosis) and phase E formed as a result of topotaxic reaction of olivine substitution. References:
 -104/T,K at P=3 kbar. The curve consists of two straight segments. The knee at 104/T,K =7.43. (t=1072oC) is accompanied by the change in the activation energy
-104/T,K at P=3 kbar. The curve consists of two straight segments. The knee at 104/T,K =7.43. (t=1072oC) is accompanied by the change in the activation energy  E=0.34 eV (the activation energy E is proportional to the slope angle) and corresponds to the glass transition temperature Tg. A jump-like activation energy change upon cooling and repeated heating occurs nearly at the same temperature but the
E=0.34 eV (the activation energy E is proportional to the slope angle) and corresponds to the glass transition temperature Tg. A jump-like activation energy change upon cooling and repeated heating occurs nearly at the same temperature but the  E value can vary depending on the direction and the rate of the temperature change.
E value can vary depending on the direction and the rate of the temperature change. E value diminishes as the pressure is increased, fig.2. At pressures 12 and 15 kbar the knee on the temperature vs the electric conductivity curve can no longer be fixed which indicates degeneration of the glass transition temperature Tg with the growing pressure. The disappearance of the Tg transition implies that the 'frozen' atomic configuration persists in the glass throughout the temperature range until its crystallization. Therefore, the data on the structure of quenched glasses cannot reflect the silicate melt structure under high pressures. The dependence of the glass transition temperature on the pressure was derived from the electric conductivity measurements results, fig.3.
E value diminishes as the pressure is increased, fig.2. At pressures 12 and 15 kbar the knee on the temperature vs the electric conductivity curve can no longer be fixed which indicates degeneration of the glass transition temperature Tg with the growing pressure. The disappearance of the Tg transition implies that the 'frozen' atomic configuration persists in the glass throughout the temperature range until its crystallization. Therefore, the data on the structure of quenched glasses cannot reflect the silicate melt structure under high pressures. The dependence of the glass transition temperature on the pressure was derived from the electric conductivity measurements results, fig.3.
# Trusov S.V., Pletchov P.Yu. and Kotelnikov A.R. Melt inclusion synthesis in alkaline feldspars of Q-Ab-Or system.
key words [melt inclusion feldspar]
The aim of this work was a melt inclusion synthesis during the process of new phase growth on natural crystals of similar composition. Inclusions were trapped with subhedral growing of crystal single blocks or within-growing crystal rims. The synthesis of melt inclusions was carried
# This work was been supported by the RFBR, Project N0 96-05-64911
27
out with the alkaline feldspar growth in the subliquidus area of the granite system under gradual temperature decrease. Mixture composition corresponded to feldspar liquid-solid equilibrium under a temperature of 780oC (Q - 20%, Ab 56%, Or - 24%).
Grains of natural Or(fraction 0,5-1 mm) were used as nuclear centers for crystal growth. Mixture with grains of natural Or was heated to 810oC (approximately by 50oC higher than the liquidus temperature) and exposed under these conditions for 4 hours (up to complete melting and homogenization of mix). Then the temperature was reduced to subliquidus during next 6 days with decreasing the rate to 10 o per day.
Fig.1.
There are particularly melting and strongly rezorbed grains of Or, which are overgrown by newly-formed alkaline feldspar (fig.1). New formations of feldspar are presented by block crystal with radial direction to large Or crystals. Via step-by-step temperature decreasing, crystals have got clearly expressed composition zones. Later (outer) zones are enriched in Na and depleted in K as compared with earlier (inner) zones of newly-formed alkaline feldspar.
Fig. 2 shows the results of chemical analyses of experimental products. Compositions of artificial melt inclusions are situated on the cotectic line or above it (in the Q field). Trapping of inclusions took place in the feldspar crystallization field. The composition of captured melt is on the line, which connected feldspar composition and initial mixture composition. Such effect is observed at feldspar crystallization on the inclusion walls. That is why some compositions of glass inclusions, which were captured in the feldspar field, are on the cotectic line.
Fig.2.
The other inclusions compositions are located in the crystallization field of Q. Such effect can be described at further feldspar growth, after the system comes to the cotectic. Q can not crystallize because one it has not nucleation centers.
Thus, we showed the principal possibility of the melt inclusions synthesis in the process of new formation growth on nuclear centers. This method is easier than crack-forming synthesis methods [2]. It allows one to observe the evolution of melt composition by the study of melt inclusion compositions in the different newly-formed zones.
References:
28