IV. Metamorphism and geodynamics
(Leaders Prof. L.L.Perchuk, Dr.Sci. V.I.Fonarev)Perchuk L.L. and Gerya T.V. PT-paths as MIRROR of dynamics of granulite complexes
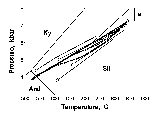
PT-paths are powerful tools in understanding the geodynamic processes, the relationships between granulite facies terrains (GFT) and greenstone belts (GSB), in particular. In recent years the formation of the Precambrian granulites have been considered within a collisional model, independently of the evolution of associated GSB. A collisional model may be applicable to those GFT that are characterized by decompression-cooling PT-paths only. However, many GFT preserve evidence for both the decompression-cooling and the isobaric cooling regimes (Fig.1). The latter results from convection of material within high temperature granulite diapir caused by its interaction with undergone cool greenstones [1]. Numerical modeling of such processes have been used to test a model of gravitational redistribution in the Earth Crust initiated by mantle derived fluid-heat flow [2, 3]. This modeling has been conducted along with calculating the PT-paths for contrasting in metamorphic conditions rocks such as granulites and greenstones. Indeed, available data show that GFT are hotter, more felsic and younger than the cratonic rocks against which they are tectonically juxtaposed. Metamorphic zoning of the cratonic wall rocks adjacent to GFT is also a well-established phenomenon. The theoretical background for numerical modeling is based on the model of gravitational re-distribution of rocks in the GSB involving a chain reaction mechanism as a result of mantle derived heat/fluid flow [3]. This model was also tested through careful field and laboratory studies of metamorphic zoning across the contacts of GSB with GFT in several crustal GFT. The Lapland complex in the Kola Peninsula [4], the Kanskiy complex in the Yenisey Range [5], and the Limpopo GFT of southern Africa [1, 6] are among them. Geological and petrological data provide evidence for the existence of a hot granulite diapir that could have resulted from significant acceleration of gravitational redistribution of the rocks of GSB due to rhythmic interlayering of initial lithologies [2]. This resulted in a chain reaction mechanism during the relatively small scale convective processes. A finite-differences numerical method was used to solve the continuity equation, Navier Stocke's equation and the thermal equation for a Newtonian incompressible fluid medium. The most exciting results were obtained from the Limpopo GFT. Fig.2 demonstrates a scenario for contrary directed movement of granulite diapir and the associated dipping (left side) of the upper crust of a GSB. with a rate of about 2.7 mm/year.
Fig. 1. PT-paths calculated from mineral geothermobarometry (solid lines) and hydrodynamic modeling (dashed lines) for metapelites of the Limpopo GFT.
Diagram a shows common decompression cooling regimes, while diagram b reflects isobaric/subisobaric cooling regimes that are typical for near contact areas of granulite facies terrains with greenstone belts.
The calculations of scenario shown in fig.2 were done using the following initial and boundary conditions: density (g/cm3): sediments - 2.7, metapelites etc. (red) - 2.8; metabasites - 3.0, komatiites (green) - 3.3, viscosity contrast - 102; isobaric cooling limits -  T=1000; boundary conditions: at top Ttop = 300, K, dVõ=dVy=0, at bottom T = 1200, K, and Võ=Vy=0, at walls dT/dx= 0, dT/dy = const., and VY=Vy=0.
T=1000; boundary conditions: at top Ttop = 300, K, dVõ=dVy=0, at bottom T = 1200, K, and Võ=Vy=0, at walls dT/dx= 0, dT/dy = const., and VY=Vy=0.
26
Fig.2. Results of numerical modeling of upward movement of a granulite diapir (light gray body) generated under lower crustal conditions and associated dipping of an upper crustal GSB composed of komatiites (black), metabasalts (dark gray), rhyolites or/and tonalites (light gray) and sediments (white). Such relationships are observed from the PT-paths deduced for the Limpopo granulite belt and the cratonic GSB wall rocks adjacent to the belt. Two wait markers indicate movement upward (to the level about 7 km) of two samples from the lower crust. The markers are tracing isobaric cooling at the level about 16-17 km (upper left marker) regime and decompression/cooling regime (lower right marker).
References:
#Fonarev V.I., Touret J.L.R., and Kotel'nikova Z.A. Fluid inclusions in rocks of the Central Kola Archean Granulite Area (Baltic shield).
Fluid inclusions were studied in the metamorphic rocks of the Central Kola Archean granulite area (Baltic Shield). The following results were obtained (Fonarev et al., 1998).
1. Studied samples contain different kinds of fluid inclusions: N2-rich inclusions with a CH4-impurity up to 7-9 mol %; CH4-rich inclusions; CO2-rich inclusions with N2 content 5-35 mol %; brine inclusions: highly concentrated CaCl2 solution (21.8-28.3 wt. %), concentrated NaCl solution (9.9-30.5 wt. %), and slightly concentrated NaCl solution (0.8-6.1 wt. %); and late secondary inclusions containing almost pure water. Such a wide distribution of N2-rich and CaCl2-rich inclusions is unknown in other granulite regions and was first discovered in this area.
2. CO2-rich inclusions vary widely in density because of their condensation or/and a partial fluid loss depending on physical and mechanical properties of host minerals. The change of N2 content from 35 to 5 mol % increases the homogenization temperature (Th) from -45.4 to -17oC and melting temperature (Tm) from -61.2 to -57.7oC.
3. Pure N2-inclusions and CO2-rich inclusions with the CO2-content ranging up to 35 mol % were only found here in banded iron formations (BIF) and associated enderbites. We suggest that during the metamorphism nitrogen was supplied from BIF, which probably were the source of CaCl2-fluids as well.
4. The metamorphic evolution of the Central Kola granulite area included three stages (Fonarev et al., 1993): M1 (670+20oC, 5.1+0.5 kbar), M2 (565+15oC, 4.0+0.5 kbar), and M3 (500+20oC, 3.2-0.4 kbar). Discrete groups of fluid inclusions were distinguished, which characterize different metamorphic stages. Special investigations of zonal (heterogeneous) garnets containing primary fluid inclusions enabled the precise determination of the temperature of fluid entrapment for one of these groups by methods of mineralogical thermometry. The estimated temperature (560oC) corresponds to metamorphic stage M2. This stage probably gave birth to the highly concentrated NaCl solutions, which were responsible for the high fluid activity which resulted in increasing iron content of garnet.
5. Data obtained suggest that N2-, CO2-, and CaCl2-inclusions were simultaneously formed during metamorphic stage M1. The fluid evidently consisted of immiscible mixture of N2, CO2, and CaCl2, even at the parameters of peak metamorphism (670oC and 5.1 kbar).
6. High-density inclusions with molar volumes (mV) equal to 42.0 cm3/mol (concentration N2 no more than 10 mol %) and 44.2 cm3/mol (concentration N2 no more than 3 mol %) were found. Formation of such inclusions suggests two successive events with subisobaric cooling of rocks at pressures 5 and 4 kbar respectively (see figure). Each event was completed with an abrupt decompression (uplift). Moreover, the original fluid, which corresponds to different metamorphic conditions, is represented by CO2-rich inclusions with N2-content ranging up to 35 mol % and mV=45.6 cm3/mol (M1) and those with N2-content about 3 mol % and mV=46.7 and 51.2 cm3/mol (M2). The relatively low density of these inclusions at these P-T metamorphic conditions is the result of water loss from
# This wark was supported by thhe RFBR (Grant N 98-05-64563) and INTAS (Project 94-2466).
27
them after their formation. For stage M1, XH2O was determined from isochore position at 0.2-0.25, which is in agreement with the values estimated by mineral assemblages (Fonarev et al., 1991). For stage M2, XH2O was estimated at 0.35, which seems too low for these P-T parameters and requires further refinement.
7. The results of this study indicate that the metamorphic evolution of granulite regions was more complex than the one-stage process of subisobaric cooling and subisothermic decompression adopted by many petrologists. The fluid inclusion studies confirm the previously proposed (Fonarev, et al., 1998) model of metamorphic evolution which implies a cyclic alternation of quite periods (subisobaric cooling) and short activization periods of regional uplifting.
Fig.1. PT-paths of metamorphism in the Archean Central Kola granulite area according to mineralogical thermobarometry and fluid inclusion investigations. Thick lines (I-VI) - isochores (mV, cc/mol) of (CO2+ N2 fluid): (I) 42.0 ( Th =-31.5°C), (II) 45.6 (Th =-45.4°C), (III) 44.2 (Th = -14.7°C), (IV) 46.7 (Th =-6.5), (V) 51.2 (Th = 6.8), (VI) 53.0 (Th = -153.4). Solid thick lines with solid arrow: supposed P-T metamorphic path. Open arrow: general trend of metamorphic P-T parameters. M1-M3: discrete stages of metamorphism. +/- d - pressure differences between of the metamorphic events and the isochore positions. Average fluid composition (mol %): (I) CO2 (93)- N2 (7), (II) CO2(65) -N2 (35), (III-V) CO2 (97)- N2 (3), (VI) N2 (100).
References:
#Fed'kin V.V. Metamorphic processes in the continental crust oceanic crust conjugation zone of Serbia.
Evolution of the physicochemical conditions of metamorphism of two geologically contrast fragments of the earth's crust (oceanic and continental types) was comparatively studied in their conjugation zone. Thermodynamic conditions of metamorphism were estimated by methods of paragenetic analysis of mineral associations, by detailed microprobe analysis of major rock-forming minerals, and mineralogical geothermobarometry based on the experimental mineral equilibria data for the middle grade metamorphic rocks. The mineralogical tool system includes the following geothermometers and geobarometers for various facial complexes of metamorphic rocks:
- Cpx-Grt geothermometer [12] and
- Cpx-Pl-Qtz geobarometer [11].
- Am-Pl geothermometer [3];
- Cpx-Pl-Qtz geobarometer [11].
Two opposite trends in the evolution of metamorphic parameters were found in the process of petrologic studies of metamorphic complexes of Serbia. Mineral associations of Pre-Caledonian amphibolites from the Serbo-Macedonian Composite Terrane (SMCT) in the Batocina region demonstrated traces of progressive metamorphism of different depth level from T=430-530oC and P=5-8 kbar to 620-680oC and P=9-10 kbar. Later retrograde alterations in the compositions of coexisting minerals of staurolite schists from the Batocina complex are possibly connected with the Alpine cycle of orogeneses. They fix the parameters in the range from epidote-amphibolite (T=540-580oC, P=8-9 kbar) to green schist (T=340-420oC , P=0.4-1.5 kbar) facies (fig.1).
# The work was supported by the RFBR, grant N 98-05-64002.
28
Fig.1. Metamorphic evolution of amphibolites (a) and metapelites (b) from the Batocina complex (BC) based on the data of mineralogical geothermobarometry. BC stands for the initial ,metamorphic conditions; Ia, Ib, Ic, Id are subisobaric prograde P-T paths; arrows changes of P-T paths for individual mineral pairs from the cores (filled symbols) to rims and contacts (open symbols).
The opposite trend of the metamorphic processes revealed in rocks of the same complex is related to the fact that rock-forming minerals possess different capability of retaining their composition and fixing the mineral formation conditions at different metamorphic stages. Nevertheless the both trends of metamorphic evolution of the complex lead in the end to a common trend of the P-T paths of mineral formation at its final (post-mesozoic) stage that, in our opinion, reflects the course of the geothermal gradient line of the region at this period.
Prograde metamorphism in the contact aureols of ultramafic bodies (oceanic crust fragments in the subduction zone, situated in the Dinaridic Ophiolite Belt) follows to formation of Grt-Cpx-Hb-Pl crystalline schists and garnet amphibolites.
Prograde zoning in central parts of rock forming mineral grains likely to be caused by highest grade metamorphic stage when a hot ophiolite slab with ultramafic rocks at its base was overtrusted onto the host continental complexes. This process was associated with the closing of oceanic realm in Middle Jurassic and collision of the continental blocks.
The rim composition of the coexisting phases (Grt, Cpx, Hb) fix the retrograde conditions of mineral formation, the T-P parameters decrease in this case from 740-830 oC and 8-10 kbar to 570-650oC and 3.5-7.0 kbar (fig.2).
Fig.2. P-T conditions for the formation of garnet amphibolites and Grt-Cpx rocks at the Bistrica contact aureole: BUB P-T initial conditions; Ia, Ib, Ic, Id subisobaric trends of Peak geotherm.
The retrograde wave reaches in its final phase the same parameter values (geothermal gradient line) as the Batocina complex, but in the opposite direction i.e. from the side of higher T and P values (fig.3).
Fig.3. Summarized data on the metamorphic evolution of the earth's crust fragments in the conjugation zone of the oceanic and continental crust. BC - initial metamorphic conditions, prograde and retrograde P-T paths (open arrows) of the Batocina complex, SMCT continental crust fragment; BUB initial metamorphic conditions and retrograde P-T paths of the Bistrica Grt-Cpx amphibolites, DOBT oceanic crust; II and III retrograde and prograde P-T paths of the Bt-Grt and young dyke rock (Grt amphybolite) form the Southern part of the SMCT continental crust fragment.
So, the evolution of metamorphic and geotectonic events of the region was for the first time represented as a unified quantitative model of evolution of the T-P parameters of deep mineral formation; the model combines prograde metamorphism of northern regions of Serbo-Macedonian Composite Terrane and retrograde metamorphism of the ophiolitic plate forced onto the Paleozoic base in the southern part of the region in question. The revealed trends of metamorphic evolution of the two geologically different earth's crust fragments lead to the same physico-chemical conditions of the final stage of the geological history of the regions evolution as a whole the P-T trend of which reflects the geothermal gradient line position. The obtained data suggest that in the post-Mezozoic time the earth's crust fragments of the Western Balkan, Peninsula, although being different in age and in structural
29
geologic position, had similar metamorphic transformation conditions and followed the same position in terms of geothermal gradient
References:
Safonov O.G. Role of potassium and sodium activities in the garnet formation in orthorocks from the Adirondacks (USA).
Metamangerites and metaanorthosites from the Adirondacks (USA) [3] are characterized by garnet-quartz and amphibole coronal textures at contacts of pyroxene, ilmenite and hornblende grains with plagioclase. A petrographic study of the rocks has shown that reaction textures of the Adirondacks are often accompanied by neogenic K-feldspar inside the coronal textures (Fig.1.a,b). This enables the determination of the role of K and Na in a metamorphic fluid during the formation of these reaction textures.
The following regularities have been observed in a change of the co-existing minerals composition: 1) in all the rocks studied NCa of plagioclase decreases by 1-4 mol% from the grain centers towards the contacts with garnet-K-feldspar-quartz coronas; 2) NCa of garnet increases at the contact with K-feldspar and plagioclase. This process is clearly manifested in metaanorthosites where calcium concentration in garnet varies insignificantly inside porphyroblasts whereas at their edges in contact with plagioclase and K-feldspar it grows dramatically by 5-8 mol% (fig.1b).
It has been found in metamangerites that NCa in garnet increases from the earlier generations of this mineral in rocks (small porphyroblasts in a matrix) to later ones (coronal textures) associated with neogenic K-feldspar.
These regularities demonstrate that along with the reactions:
An + 2Fs = 1/3 Grs + 2/3Alm + Qtz (1)
An + Hed = 2/3 Grs + 1/3 Alm + Qtz (2)
the growth process of garnet-K-feldspar-quartz coronas was controlled by the K and Na activities in metamorphic fluid. The co-existence of calcic garnet, plagioclase and K-feldspar inside the coronas is governed by the equilibrium
An + 3Qtz + [4/3K+ + 2/3 H2O] = 4/3 Kfs + 1/3 Grs +
+ 4/3 H+] (3)
Microstructural features of coronal textures in the studied rocks and temperature estimations of their formation showed that they had formed at the metamorphic regressive stage at temperatures 635+70oC at P = 6-7 kbar at the rock subisobaric cooling stage [1,6]. Calculations of oxygen fugacity in a fluid equilibrated with coronal minerals from the Crt + O2 = Mt + Pl + Qtz equilibrium have shown that in the temperatural range 700-600oC (P=6-7 kbar) the calculated values of -lg fO2 = 16-18 are close to the graphite+O2 equilibrium line. The absence of graphite in the studied samples implies that the metamorphic fluids contained small amounts of CO2. The low activities of H2O (0.1-0.2 [7]) in the fluid at the formation of coronal textures in metamangerites were due to considerable concentrations of potassium and sodium chlorides in the aqueous fluid (to X salts=0.7). The enrichment of the metamorphic fluids in KCl and NaCl in orthorocks from the Adirondacks is attested by chlorine-rich potassium hornblendes (NHblK = 36-48, to 1.5-2 wt% chlorine) and chlor-apatites associated with garnet. The subsolidus diagram lg(aK+/aH+)fl - lg(aNa+/aH+)fl (fig.2) illustrates the regularities of the compositional variation of the co-existing garnet and K-feldspar in equilibrium with the fluid, containing potassium and sodium salts at T=700oC, P=6.5 kbar (mean temperature and pressure values for coronas ) and aH2Ofl =0.1. The diagram also illustrates the equilibrium values of lg(aK+/aH+)fl and lg(aNa+/aH+)fl calculated from the compo
30
sitions of the co-existing garnet and K-feldspar in coronas from the examined orthorocks. The potential source of alkalis in the fluids of the Adirondacks can be syn-post-metamorphic leucocratic, fayalitic, and alaskitic granites (10-11% Na2O or 10% K2O) that surround anorthosite-mangerite massifs.
Two sequential stages have been distinguished in the fluid evolution of the Adinrondacks rocks: (1) high -temperature stage (700-600oC) when rocks are affected by aqueous fluids enriched in various salts, primarily, in K and Na salts and (2) low temperature stage (<600oC) when rocks are altered due to aqueous-carbonic fluids which gives rise to chlorite-carbonate, chlorite, and other microveins [2,5,7].
Fig. 1. A) Garnet-K-feldspar-quartz coronal texture in metamangerite; B) Garnet porphiroblasts associated with K-feldspar microveins in metaanothosite. Numbers in Kfs field - NOrKfs, fractions in Grt field - NMg/ NCa of garnet. Back-scattered image. Electrone microscope CamScan
Fig.2 The lg(aK+/aH+)fl-lg(aNa+/aH+)fl diagram for Grt+Opx+Pl+Kfs+Qtz+(K-Na-H)Cl-fluid assemblage of Adirondacks orthorocks, calculated at shown temperature, pressure, aH2O=0.1 and constant plagioclase composition. Streight lines - NOrKfs, sub-vertical lines - NCaGrt. Points on the diagram show compositions of co-existing garnet and K-feldspar in the coronas and equilibrated activities of potassium and sodium at taken parameters.
References:
31
Graphchikov A.A. Experimental study of biotite stability in assemblage with Grt, Sil, Kfs, Ilm and Qtz in the presence of H2O-CO2 fluid.
The compositions of minerals and the stability of the assemblage biotite + garnet + sillimanite + sanidine + quartz + ilmenite + water-carbon dioxide fluid (Bt + Grt + Sil + Kfs + Qtz + Fl) have been experimentally studied. The runs were performed with preliminarily synthesized minerals at T=750oC, P=5 and 3 kbar, molar fraction of water in the fluid X(H2O)-0.5 and oxygen fugacity given by the NNO buffer. An analysis of the obtained products has shown that at the parameters of the runs the divariant assemblage in question is stable and the mineral equilibrium fits dehydration reactions:
KFeTiAlSi3O10(OH)2=KAlSi3O8+FeTiO3+H2O and
KMg3AlSi3O10(OH)2+2SiO2+Al2SiO5=KAlSi3O8+
+Mg3Al2Si3O12+H2O
The comparison of the results with those obtained for biotite of the phlogopite annite series in the assemblage biotite + orthopyroxene + sanidine + quartz and for titanium containing biotite has shown that in assemblage with sillimanite and garnet the field of stability of biotite of fixed iron content (Fe/(Fe+Mg)) extends towards higher-temperature region. The microprobe analysis of the mineral compositions from the products of the runs has shown that biotites are characterized by incorporation of titanium and increased concentration of excess aluminium (the occurrence of muscovite and eastonite end members). The incorporation of titanium and excess aluminium into biotite is known to expand its stability with respect to temperature. In the studied equilibrium the incorporation of titanium and the presence of muscovite and eastonite end members rendered a summary effect on an increase of the temperature stability of biotite.
The obtained data indicate that for the identical composition of the metamorphizing fluid the dehydration reactions in metabasitic rocks occur at lower T-P parameters than those in metapelitic ones.
Zharikova L.Yu. and Fonarev V.I. Zonal garnets: experimental and natural data.
Garnets consisting of two or more (3 to 5) zones of different composition (Fe-, Fe-Mg, Mn-, Fe-Mn) were synthesized (Fig. 1, Run 473). Microprobe analysis showed that each of the zones is composed of the subzone with clear zonal distribution of the above-listed elements and the 'plateau', within which the composition of the mineral is relatively constant. The thickness of the subzone with microzoning (SM) basically depends on the fluid composition. With aqueous carbon dioxide fluid, SM no more than 50 m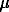 in size was experimentally obtained for three weeks. In this case, the subzone is incomplete, i.e. even rim compositions are not constant. In Run 455 (modeling of the Fe-Mg zoning with an Alm seed, duration 21 days), relatively low-magnesian garnets with iron content no more than 0.81 and above crystallized at Xfe of garnet initially set at 0.6. The size of SM varies in this case from 10 to 50
in size was experimentally obtained for three weeks. In this case, the subzone is incomplete, i.e. even rim compositions are not constant. In Run 455 (modeling of the Fe-Mg zoning with an Alm seed, duration 21 days), relatively low-magnesian garnets with iron content no more than 0.81 and above crystallized at Xfe of garnet initially set at 0.6. The size of SM varies in this case from 10 to 50 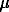 m. The maximum thickness of the newly-formed garnet zone (up to 1000
m. The maximum thickness of the newly-formed garnet zone (up to 1000 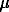 m) and, therefore, the maximum size of SM (up to 150-200
m) and, therefore, the maximum size of SM (up to 150-200 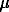 m) was obtained in the aqueous carbon dioxide-chloride solution. Poorly cut zones and broken edges of a starting crystal were welded first of all (Fig. 1). The plateau and SM are typically larger in such areas than on crystal faces. This may cause significant variations of the total thickness of the newly-formed garnet zone over the perimeter of a grain. Temperature variations within 50 degrees (700-750 oC) do not affect the SM size. In both cases, zoning appeared within the first two weeks from the beginning of the experiment.
m) was obtained in the aqueous carbon dioxide-chloride solution. Poorly cut zones and broken edges of a starting crystal were welded first of all (Fig. 1). The plateau and SM are typically larger in such areas than on crystal faces. This may cause significant variations of the total thickness of the newly-formed garnet zone over the perimeter of a grain. Temperature variations within 50 degrees (700-750 oC) do not affect the SM size. In both cases, zoning appeared within the first two weeks from the beginning of the experiment.
Fig.1. A zonal garnet crystal (Run 473). First zone - synthetic Alm, second zone - Alm-Py, third zone - Alm-Spess. First of all, poorly cut zones and broken edges of the starting grain were welded. The size of the newly-formed garnet zone varies over the perimeter of the grain.
Running experiments for a longer time (up to 1 month) resulted in the increasing plateau of constant garnet composition. Fig. 2 shows Fe, Mg, and Mn content profiles for garnet from Run 476 (four zones: Alm, Alm-Py, Alm-Spess, and Alm). In the second zone of Alm-Py composition, magnesium appears in significant amounts at the distance of 2-3 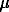 m from the boundary, and the Mg content gradually increases towards the boundary with the next Fe-Mn zone. In this zone, magnesium is absent (even within 2-3 m
m from the boundary, and the Mg content gradually increases towards the boundary with the next Fe-Mn zone. In this zone, magnesium is absent (even within 2-3 m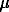 from the boundary between the zones), and no Mg traces were found even in longer runs. No significant amounts of magnesium were found in the garnet seed after all successive runs of a total duration more than 60 days. Manganese showed somewhat different behavior. This element is present in small amounts in the second Fe-Mg zone at the distance of 10-20 m
from the boundary between the zones), and no Mg traces were found even in longer runs. No significant amounts of magnesium were found in the garnet seed after all successive runs of a total duration more than 60 days. Manganese showed somewhat different behavior. This element is present in small amounts in the second Fe-Mg zone at the distance of 10-20 m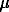 from its boundary and in the pure-iron (fourth) zone of a garnet crystal approximately at the same distance from its boundary.
from its boundary and in the pure-iron (fourth) zone of a garnet crystal approximately at the same distance from its boundary.
32
Fig.2. Fe,Mg, and Mn content profiles across a zonal garnet grain (Run 476). Four zones: Alm, Alm-Py, Alm-Spess, and Alm. (1) Fe-profile across zones 1-4; (2) Mg-profile across zones 2-3; (3) Mn-profile across zones 2-4.
33
Fig.3. A fragment of the boundary between two zones of different composition (Alm and Alm-Py). Images in secondary electrons. Signs of Alm dissolution are visible.
Fig. 4. Compositional profiles (f=Fe/Fe+Mg) across garnet: (a) Sample 15/310.4 (profiles 2-16 and 17-29, see insert); (b) Sample 7g (profiles 1-36 and 37-58, see insert). Temperature estimates by Bt-Grt and Crd-Grt thermometers are indicated for both samples.
Obtained data illustrate the mechanism of growth of zonal garnet during the dissolution-crystallization process (dissolution: starting components, seed; crystallization: newly-formed garnet). The dissolution of the starting phases is indicated by the correspondence of newly-formed crystals to the starting phases in composition, which was observed in all runs. The dissolution processes were also established by scanning electron microscopy during the detailed study of the garnet compositions closely to the boundaries between the different zones (Fig. 3). In this process, no significant diffusion of components took place in the solid phase (at least Mg is absent in the garnet). However manganese behavior is not completely understood yet. Data obtained can be interpreted either in terms of diffusion or with the dissolution-crystallization mechanism. The latter should be applied to the zones of small defects at crystal edges (pores, microcracks, etc.), which cannot be detected by the aforesaid analytical methods. This mechanism seems to be most likely, however the problem calls for further investigation.
Detailed microprobe studies of zonal garnets from metamorphic rocks of the Central Kola Archean granulite area (Fig. 4) confirmed our experimental results. For one sample (15/310.4), the iron content of garnet is almost constant (0.65-0.66) in the cores and typically increases towards the rims at the contact with biotite (up to 0.71-0.72) and rarely with plagioclase (up to 0.68-0.69) and cordierite (up to 0.673). Usually, the garnet composition does not vary from the cores to the rims contacting with plagioclase and cordierite. The temperatures of crystallization of minerals in this sample obtained by Grt-Bt and Grt-Crd geothermometers are in good agreements (555 and 570oC) and correspond to the second stage (M2) of regional metamorphism. These data testify that garnet was completely recrystallized at this stage, and its zoning resulted from changing fluid composition and activity, rather than temperature changes, as it usually happens. The iron content of the garnet contacting with biotite, cordierite, and plagioclase increased at isothermic conditions.
Another sample (7g) is characterized by the clearly defined zonal structure: Mg-rich core (f = 0.74-0.75) and Fe-rich rim (f = 0.79-0.82) at the contact with plagioclase. The boundary between the zones is very clear and evidently formed as a result of crystallization of the new garnet from Fe-containing fluids rather than as a result of diffusion. The iron content of the mineral is even higher (0.86) at the contact with the most Mg-rich biotite, which is present in the sample in three modifications of different composition. Mineralogical thermometers applied to garnet and biotite of corresponding compositions indicated parameters of the whole thermal history of metamorphism in the region: T=660oC (cores, M1), T=565oC (newly-formed Fe-rich zone, M2), T=490oC (contacts with the most Mg-rich Ti-poor biotite, M3).
+Gerya T.V. and Perchuk L.L. 'Geopath' database: equation of state for individual gases
Gas-liquid phase transitions are common for any individual fluid. The equilibrium line
Liq = Gas (1)
is always ended in a critical point. PT-parameters of such point were often used for calculations of thermodynamic properties of individual gases (a method of corresponding states). Existence of the critical points, however, reflects not only simple deviation of a fluid from an ideal gas. They rather show the order-disorder in statistics of distribution of gas-like and liquid-like particles in the fluid structure [1]. This allows calculation of molar fractions of such particles in a fluid using PVT experimental data along the phase transition (1) line, and then evaluation of PVTXLiq .
34
Table 1. Calculated parameters from eqn. (2) for Gibbs Free Energy for some pure fluids
|
Parameters |
Units |
Ar |
CH4 |
CO2 |
H2O |
|
|
cal/mole |
247.62019 |
1479.8541 |
4925.8228 |
10717.479 |
|
|
cal/mole/K |
30.374805 |
28.631362 |
33.086146 |
29.255727 |
|
|
cal/mole/K |
0.1839337 |
-0.345089 |
-2.400152 |
-5.135704 |
|
W1H |
cal/mole |
-5467.852 |
-4982.556 |
-4985.791 |
-6882.225 |
|
W1S |
cal/mole/K |
9.1261191 |
7.4123812 |
8.8117827 |
-2.797644 |
|
W1Cp |
cal/mole/K |
3.9236457 |
1.5429817 |
0 |
1.2155956 |
|
Ho |
cal/mole |
1224.7253 |
3137.3241 |
3550.8439 |
340.86125 |
|
So |
cal/mole/K |
6.6004625 |
15.925436 |
17.917433 |
1.0526741 |
|
|
cal |
15.232284 |
580.55343 |
1350.55 |
1096.2692 |
|
|
cal/bar4/5 |
0.0387218 |
0.6159146 |
0.5724886 |
0.0591027 |
|
c1 |
0.4177045 |
1.0182855 |
2.1126283 |
7.2357551 |
|
|
O s |
cal/mole/bar4/5 |
3.4641468 |
4.3967565 |
4.4923383 |
2.3496757 |
|
|
bar |
8385.2962 |
5444.5446 |
6551.3123 |
6208.8678 |
|
c2 |
2.0115894 |
4.3424714 |
5.7876907 |
0.3148243 |
|
|
|
% |
0.30 |
0.54 |
0.50 |
0.48 |
|
|
% |
2.01 |
2.59 |
3.27 |
2.27 |
|
|
cal/mole |
6.6 |
14.3 |
10.3 |
12.2 |
|
|
cal/mole |
24.3 |
45.3 |
29.6 |
38.4 |
|
|
cal/mole/K |
0.06 |
0.31 |
0.11 |
0.17 |
|
|
cal/mole/K |
0.56 |
1.28 |
0.82 |
0.86 |
|
|
% |
0.39 |
0.35 |
0.51 |
0.46 |
|
|
% |
1.39 |
1.41 |
1.16 |
1.99 |
The diagram has to be very similar to that of a mixture with an immicibility gap (Fig.1). Thus, theory of non-ideal solutions can be used for derivation of equation of state for a fluid. Fig.1 exemplifies clearly that XLiq in supercritical field is a function of P and T only. The diagram also shows a similarity of a fluid system to the solid state highly ordered systems such as alkali feldspars.
This similarity allowed to develop a structural-thermodynamic model and derive the general equation of state for a fluid [3]:
G = min = Ho - TSo + RT[XLiqlnXLiq + XGaslnXGas] + XGas G00 + XGasXLiqWG1 + XGasRTln(
G00 + XGasXLiqWG1 + XGasRTln(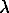 +P) + c1RTln[1-exp(-
+P) + c1RTln[1-exp(- Gs/RT)] + c2RTln[1-exp(-
Gs/RT)] + c2RTln[1-exp(- Hs/RT)] +
Q
sB - (c1+c2){
Hs/RT)] +
Q
sB - (c1+c2){ Hs(1-T/To)exp(-
Hs(1-T/To)exp(- Hs/RTo)/ [1-exp(-
Hs/RTo)/ [1-exp(- Hs/RTo)]+RTln[1-exp(-
Hs/RTo)]+RTln[1-exp(- Hs/RTo)]}, (2)
Hs/RTo)]}, (2)
where 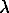 =
= 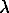 2XLiq2,
2XLiq2,  Gs=
Gs= Hs+
Hs+ Q
sB, B=5/4[(P+
Q
sB, B=5/4[(P+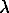 2)4/5-(Po+
2)4/5-(Po+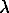 2)4/5], XGas+XLiq=1,
2)4/5], XGas+XLiq=1,
 G00=
G00= H00 -T
H00 -T S00 +
S00 +  Cp00[T-To-Tln(T/To)], WG1=WH1 -TWS1 +WCp1[T-To-Tln(T/To)],
Cp00[T-To-Tln(T/To)], WG1=WH1 -TWS1 +WCp1[T-To-Tln(T/To)],
where R=1.987 cal/mole/K, To=298.15 K, Po=1 bar. G = min is the condition of equilibrium stability of an ordering fluid at given T and P. The above parameters for some gases are given in Table 1. These parameters were obtained for 1 bar> P > 10 kbar and 100 K>T>1300 K. However eqn.2 shows very strong extrapolation ability, reproducing independent high-pressure and high-temperature experiments up to 1 Mbar and 2000oC.
Equation (2) seems to be very effective for polar (H2O, CH4, CO2), as well as non-polar gases (Ar etc.). Mean square uncertainty of description of experimental data are as follows: 7-14 cal/mole - for the Gibbs energy, 0.30-0.54% - for volumes, 0.06-0.31 cal/mole/K - for entropies, and 0.35-0.51% - for pressures along the phase transition line (1). The eqn. (2) is efficient for petrological databases and we used it for GEOPATH [2].
35
References:
Gerya T.V. A new empirical amphibole geothermobarometer
Amphibole is a common mineral of metabasic rocks from green schists to granulite facies. Perchuk [1] was the first to calibrate the amphibole-garnet and amphibole -plagioclase geothermometers, which have been widely used in years. Plyusnina [2] calibrated experimentally the amphibole-epidote-plagioclase-quartz geothermobarometer well used by metamorphic geologists during last ten years. Many empirical and semi-empirical calibrations of amphibole thermometers and barometers are listed in Spear's book [3]. In many cases, however, applications of the instruments for measuring the PT-parameters in amphibole-bearing rocks show large non-systematic deviations from estimates of PT-parameters in intercalating metapelites. On the other hand, it is well known that the amphibole composition changes systematically with degree of metamorphism. This particularly concerns the Al and Si content in amphibole reflecting several internal reactions in very complex amphibole structure [3]. Taking into account this regularity we evaluated a new empirical calibration of monomineral amphibole geothermobarometer. For this purposes we used experimental data [2], and some data on correct determination of temperature for amphibole-bearing but garnet-free rocks in their intercalation with metapelites, for which geothermometers (Crd+Grt, Bt+Grt, Chl+Grt) are well calibrated [4]. Thus, combination of Plusnina's data with empirical measurements allows calibration of an uniform amphibole geothermobarometer. The best fit for 48 experimental and 32 natural amphiboles from garnet-free assemblages yields the following eqs:
T,K= (6119 - 28.4P + 114XMg) / [8.181 Rln . (8.489 -
-CSi)] (1)
P ,kbar= [2543 - 4.744T + 175XMg + R.T ln.(CAl +
+ 0.433)] /148.1 (2)
where XMg = Mg/(Fe+Mg), CSi and CAl are coefficients in amphibole formula calculated for 13 cations. Simultaneous solution of eqns. (1) and (2) as a system of the second order yields the equilibrium PT-parameters. Precision is estimated as much as +37o and +1.2 kbar. An application of this thermobarometer to garnet-bearing rocks shows a systematic increase in pressure up to +2 kbar, if amphibole is in a direct contact with garnet.
Fig.1. Monomineral amphibole geothermometer calculated with equations (1) and (2) for garnet-free rocks. A and c are coefficients at Si in amphibole formula, calculated for 13 cations at XMg=0 and XMg=1, respectively; b and d are coefficients at Al in amphibole formula, calculated for 13 cations at XMg=0 and XMg=1, respectively.
References:
#Konilov A.N. Graphchikov A.A., Fonarev V.I., Sultanov D.M. A consistent system of geothermometers and geobarometers: testing with using independent experimental data.
The testing results of early proposed consistent system of geological thermometers and barometers [1-4] are presented here. More than 700 experiments reported in 57 recent publications (1989-1998) were used for this testing. Experimental conditions and the statistical parameters describing deviations of the calculated temperatures or pressures are given in the table 1. For Grt-Cpx equilibrium the results of 224 experiments from 26 publications are compared with 125 experiments of Aranovich and Pattison [5].
The Opx-Cpx geothermometer [2] is based on the experiments in system CFMS. This stipulated some restrictions at them usage. The testing in systems with more number of components has shown a possibility of its wider application. Comparison of Opx-Cpx, Grt-Opx and Grt-Cpx geothermometers carried out at the base of 32 experiments show very high correlation of their estimations (Table 2). The Grt-Crd geother
# This work has been supported by the Russian Foundation for Basic Research Grants 98-05-64569, and 98-05-64564, and INTAS Grant 94-2466.
36
mometer have a weak correlation with the temperature of the experiments, however, have small average value of  T, which is much lower than the standard error (s). It was showed earlier [1,2] that the geothermometers of consistent system are better than the other versions corresponding to independent experiments having been available at that moment, except Grt-Bt geothermometer, for which such experiments were not quite enough. The testing has confirmed a high reliability and consistency of the two-pyroxene and garnet-pyroxene geothermometers' data in a wide range of compositions and P-T conditions and reliability of the garnet-cordierite geothermometer. The Grt-Bt geothermometer included into a consistent system describes unsatisfactorily the new experimental data at temperatures more then 700 oC (Fig.1). It requires the critical evaluation both of the experimental data used at testing and the existing versions of the Grt-Bt geothermometer.
T, which is much lower than the standard error (s). It was showed earlier [1,2] that the geothermometers of consistent system are better than the other versions corresponding to independent experiments having been available at that moment, except Grt-Bt geothermometer, for which such experiments were not quite enough. The testing has confirmed a high reliability and consistency of the two-pyroxene and garnet-pyroxene geothermometers' data in a wide range of compositions and P-T conditions and reliability of the garnet-cordierite geothermometer. The Grt-Bt geothermometer included into a consistent system describes unsatisfactorily the new experimental data at temperatures more then 700 oC (Fig.1). It requires the critical evaluation both of the experimental data used at testing and the existing versions of the Grt-Bt geothermometer.
Table 1. Comparison of measured and calculated temperatures and pressures using different geoothermometers and geobarometers.
|
Sensors |
N1 |
N2 |
P, kb |
T,°C |
Av. |
 |
R1 |
R2 |
|
Opx-Cpx |
25 |
117 |
0.001-60 |
875-1650 |
-32 |
100 |
0.825 |
0.609 |
|
Grt-Opx |
22 |
76 |
5-110 |
850-1950 |
12 |
134 |
0.879 |
0.060 |
|
Grt-Cpx |
26 |
224 |
10-140 |
850-2035 |
-37 |
91 |
0.902 |
0.449 |
|
Grt-Cpx1) |
1 |
125 |
15-29 |
800-1200 |
-66 |
133 |
0.630 |
0.355 |
|
Opx-Cpx, Grt-Opx and Grt-Cpx |
8 |
34 |
10-60 |
900-1650 |
-282) |
952) |
0.8942) |
0.4742) |
|
Grt-Crd |
3 |
51 |
5-12 |
800-1050 |
-35 |
84 |
0.476 |
0.249 |
|
Grt-Bt |
12 |
106 |
2-34 |
550-1225 |
122 |
90 |
0.626 |
0.260 |
|
GOPQ |
6 |
28 |
5-17 |
850-1000 |
-0.26 |
1.59 |
0.843 |
0.214 |
|
GCPQ |
5 |
21 |
10-20 |
850-1000 |
-0.35 |
1.36 |
0.900 |
0.277 |
|
GOPQ and GCPQ |
2 |
4 |
10-15 |
900-1000 |
-0.303) |
1.083) |
||
Note: N1 - number of publications; N2 - number of experiments; Av. - average value of Texp.-Tcalc. (=DT) or Pexp.-Pcalc. (=DP); s - standard deviation of DT or DP; R1 - correlation coefficient of Texp.(Pexp.) and Tcalc.(Pcalc.); R2 - correlation coefficient of Texp.(Pexp.) and DT (DP); 1) Data of [13] only; 2) given Tcalc. is the average of temperatures calculated from three thermometers;.3) given DP is the difference between the two geobarometers.
Table 2. Correlation matrix
|
TOpx-Cpx |
1 |
|||
|
TGrt-Opx |
0.934 |
1 |
||
|
TGrt-Cpx |
0.966 |
0.916 |
1 |
|
|
Texp. |
0.879 |
0.838 |
0.912 |
1 |
Fig.1. Comparison of garnet-biotite thermometry utilizing experimental data. Data from: 1 - 11 undivided publications; 2 - experiments in which XFeGrt > 0.98 [11]; 3 - [9]; 4 - [6]. The solid lines delineate a 1:1 correspondence between calculated and experimental temperature. The trend-line (dashed) was obtained using of data [9] only.
37
It is clearly seen (Fig.1) that the new experiments carried out by Gessmann et al. [6] using Ferry and Spear’s design provided results that correspond better to our recommendation (calibrations of Perchuk and Lavrent'eva [7] and Holdaway and Lee [8], averaged) than to those of Ferry and Spear [9]. At temperatures near 600°C the divergence between results from various series of experiments is minimal.
The GOPQ and GCPQ geobarometry are in the nice agreement with the new experiments (Fig.2). Four of them contains the assemblage Grt-Opx-Cpx-Pl-Qtz. The pressures calculated with using two geobarometers have an average divergence only 0.3 kbar.
The calculations were carried out using the program TPF that represents a database containing more than 400 versions of geological thermometers, barometers and oxygen barometers for more than 80 mineral equilibria [10]. The considered systems of geological sensors are allocated in a special section. The other variants of these sensors are also available in the TPF program.
Fig. 2. Comparison of garnet-pyroxene (Grt-Opx-Pl-Qtz and Grt-Cpx-Pl-Qtz) barometry utilizing experimental data from 8 publications and unpublished experimental data of J.-M. Montel and D. Vielzeuf (personal communication, 1997). Grt-Opx-Pl-Qtz geobarometer [3]: in assemblages Grt+Opx+Pl+Qtz (1), Grt+Opx+Cpx+Pl+Qtz (3) or Grt+Opx+Pl+Qtz? (5); Grt-Cpx-Pl-Qtz geobarometer [4]: in assemblages Grt+Cpx+Pl+Qtz (2) or Grt+Opx+Cpx+Pl+Qtz (4). The solid lines delineate a 1:1 correspondence between calculated and experimental pressure.
References:
Sultanov D.M., Maaskant P., Graphchikov A.A. TPF-6.0 program for calculating mineral formation parameters a new version.
We have improved the TPF-6.0 program complex for calculating mineral formation parameters using mineral sensors, namely, mineralogic geothermometers, geobarometers, and oxygen geobarometers. The data base of the program involves more than 400 equations for minerals sensors available in the literature for over 20 years. The compositions of co-existing minerals are used in the calculations. The program is extensively employed in petrologic studies and is constantly complemented by new mineral sensors which appear in publications. Several years after the program had been created it appeared necessary to update the software referring to the user interface and extension of the possibilities of processing variable-composition minerals. The proposed version has a more convenient user menu, a greater scope is provided using the mouse and for working in Windows 95. In order to use in calculations a greater set of mineral associations the mineral and elemental (set of chemical components for representation of a mineral composition ) bases of the program have been extended. For basic rock-forming minerals (pyroxenes, garnets, amphiboles, micas, etc) the corresponding part of the program has been developed for calculating formula amounts of minerals (including Fe3+ content) by different methods.
The program can be obtained gratis at the address:ftp.iem.ac.ru/pub/geology/tpf.zip.
38