 -phase) is discovered.
-phase) is discovered.Khitariada-97. ABSTRACTS.
Magmatic systems, fluid magmatic interaction, melts properties
# Litvin Yu.A., Chudinovskikh L.T., Zharikov V.A. Crystallization of diamond and graphite in mantle alkaline-carbonate melts in experiment at 7-11 GPa.
key words [diamond graphite alkaline-carbonate melts high pressures experiment]
The conditions of mantle genesis of diamond are still controversial, but the problem of the experimental reproduction of 'natural synthesis` of diamond does not look exotic. Diamond-bearing rocks, syngenetic inclusions in diamonds, and diamonds themselves have assumed great importance in geodynamic constructions [1]. Of special interest are inclusions of alkaline mineral phases-silicate [2] and carbonate [3] in diamonds. In experiment, following a synthesis of diamonds in kimberlite-graphite mixtures using a high-pressure 'multianvil split-sphere` apparatus [4] carbonate-graphite compositions [5] were successfully tested. The 'carbonate` line of the experimental modeling of the mantle diamond formation appears most prospective as carbonate melts to all appearances characterized under high pressures by a significant solubility of carbon (diamond and graphite) comparable with such in metallic melts employed in the known 'metal-graphite` synthesis of diamond [6]. The goal of this work is to study experimentally the processes of crystallization of diamond in alkaline-carbonate system K2Mg(CO3)2 -carbon and some specific features of diamond-forming alkaline-carbonate melts using original methods designed on the base of a high-pressure 'an anvil-with-hole` apparatus [7] within 7-11 GPa.
The most interesting experimental results are as follows:
1) The crystallization of diamond in alkaline-carbonate melts in the runs at 9-11 GPa and 1700oC with the duration of 10 min. In the process of spontaneous nucleation there form flat-faced transparent colorless and yellowish single crystals of octahedral habit (octahedrons and cubooctahedrons) measuring to 0.1 mm. The microprobe analysis has shown that the composition of the crystallization medium corresponds to the initial alkaline carbonate, i.e. melting of K2Mg(CO3)2 under the experimental conditions is congruent. Apparently, diamond crystallized from the carbon solution in the alkaline-carbonate melt.
2) Formation of graphite single crystal in alkaline-carbonate melts in the runs performed at 8-9 GPa and 1700oC with the duration of 5 min (fig.1). Recrystallization of starting polycrystalline graphite gives rise to black single crystals of graphite measuring to 0.2 mm. This process is accomplished under the conditions of thermodynamic stability of diamond with graphite being an unstable phase. The observable effect of metastable recrystallization of graphite is known for the 'metal-graphite` synthesis of diamond as well and is characteristic of the states of metastable supersaturation of a carbon solution in a melt (metallic or alkali carbonate) with respect to diamond [8].
3) Partial dissolution of diamond single crystals in alkaline-carbonate melts is found in the runs where well-faceted crystals of 'metal-graphite` synthesized diamond are in contact with the alkaline-carbonate melt (Fig.1 - runs at 8 GPa and 1500oC). There form characteristic spherical 'sleek` dissolution surface which is indicative of the melted state of alkaline carbonate.
4) The growth of K2Mg(CO3)2 single crystals of the octahedral habit from the alkaline-carbonate melt occurred at 8 GPa and 1350oC. There formed flat-plane colorless transparent octahedrons measuring to 0.3 mm the composition of which corresponded to the composition of the starting rhombhohedral phase. The obtained data suggest the preliminary conclusion that the earlier unknown cubic polymorphic high pressure modification of K2Mg(CO3)2( -phase) is discovered.
-phase) is discovered.
The experimental studies performed enable to draw a number of conclusions about the diamond crystallization conditions in alkaline-carbonate melt K2Mg(CO3)2. This melt is a good solvent for diamond and graphite under high pressures. Possibly, the mechanism is operative described in [9] for the 'metal-graphite` synthesis of diamond as the conception of solvent. The state of the supersaturation of carbon solution in alkali-carbonate melt with respect to diamond is due to the difference in the solubilities of metastable graphite (carbon source) and diamond can be enhanced by temperature gradients of the apparatus. The solubility of carbon and the difference in the solubilities of metastable graphite and diamond is lower in the investigated alkaline-carbonate melt than in metal-solvent melts which is realized in a relatively lower crystallization rates of diamond and in a substantially broader metastable supersaturation field (MSF) with respect to diamond (fig.1), positioned between the graphite-diamond
6
equilibrium curve and the lower boundary the spontaneous nucleation region of diamond (i.e. the field of the labile supesaturation (LSF) of carbon solution in the melt with respect to diamond).
Fig.1. Experimental conditions at high PT-parameters: 1-equilibrium curve graphite-diamond; 2 - PT-dependence of the eutectics of the K2Mg(CO3)2 - graphite-diamond (estimation); 3-the boundary between metastable oversaturation region of carbon solutions in alkaline-carbonate melt in relation to diamond (MSF) and region of labile solutions (LSF); 4 - experiments on diamond crystallization; 5-experiments on the crystallization of metastable monocrystal graphite; 6-experiments on partial dissolution of single diamond crystals in an alkaline-carbonate melt and formation of cubic K2Mg(CO3)2 modification.
It was found that diamond crystallizes effectively in the melts of the systems Na2Mg(CO3)2-C and NaKMg(CO3)2 -C as well. Both spontaneous nucleation and seed growth were established.
References:
# Epelbaum M.B., Simakin A.G. Experimental study of conjugated degassing and crystallization of a magmatic melt.
key words [melt degassing experiment]
As we know from publications, melt degassing upon decompression is the matter of concern in two laboratories only: in the University of Jerusalem (Israel) and the University of Bayreuth (Germany). Those works report the first data obtained for the kinetics and mechanism of the gas phase segregation. Apparently, no studies have been performed on crystallization upon decompression.
Meanwhile, the processes of crystallization and vesiculation in nature proceed simultaneously and are of essential mutual effect. The manifestation of this effect can be observed both in natural and experimental samples. This is facilitation of nucleation due to the occurrence of interphase boundaries, accumulation of the gas phase at the crystallization front.
At the present time the decompression technique leaves much to be desired. There are practically no apparatus providing a gradual pressure relies for a long period and with good reproducibility of the decompression rate in time. The creation of such apparatus should be necessary. Our version of the technical solution consists in that in addition to a fine control valve (in front of it) a pressure regulator is included into the scheme. Its design is analogous to those set on gas cylinders. Several hour runs have shown that the temporal pressure change is almost linear and that the reproducibility of the regime on repeating the runs is moderately good. Now we are going on selecting fitting parameters for longer runs (several days).
We shall briefly discuss some experimental results and observations. We have experimentally observed various effects upon degassing of a water-containing granite melt at different decompression rates and different initial states of the melt. Upon degassing of a homogeneous melt there appear fronts of intensive bubbling at the sample surface, the space distribution of bubbles is inhomogeneous. Once a heterogeneous melt containing air microbubbles is degassing, the inhomogeneities in the distribution of the newly-formed bubbles arise only at a relatively great decompression rate. These bubbles are local with a scale close to the distance between the initial bubbles. The crystallization of alkali feldspar in a rhyolite melt upon degassing is observed only at its heterogeneous nucleation at decompression rates of an order of 0.5 kb a week. The Fsp crystals characteristic dimensions are about 20·50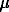 m.
m.
# Authors greatly appreciate financial support of RFBR grant N 96-05-64262
7
The size distribution of the bubbles (BSD) was studied using two techniques. Numbering of the sample surface Images was performed by scanning the photographs taken in the reflected light (Institute of Experimental Mineralogy RAS) and by recording the Images in reflected electrons directly from the electron microscope (Pisa, Italy). The sought size distribution was calculated using the program realising Schwartz -Saltykov method (Armienti et al, 1994). The validity of the results was ensured by the measurements at different magnifications with overlapping size intervals.
At a relatively slow degassing with the decompression rate of an order of 0.5 kb/week (steps 40 atmospheres each uniformly in time) the BSD in the initially microheterogeneous melt is close to the power vs size plot. At higher degassing rates (approximately 0.5 kb/day) this dependence exhibits clear local maxima corresponding to nucleation acts. The BSD was used to estimate the volumes of the segregated fluid phase. It was found that at decompression at a rate of 0.5 kb/week the amount of the liberated gas is close to equilibrium, at lower rates a considerable supersaturation of the melt with a water fluid (several times as great as the initial one) at the parameters of the completion of the run is observed.
It has been shown that the obtained distributions are, by and large similar, to the distribution of natural products of the explosive activity of the Etna (Sicily). The local coalescence of bubbles in partially decrystallized experimental samples does not significantly change the size distribution parameters. The experimental BSD differs considerably from the distribution parameters of vesicles in natural samples due to coalescence and fragmentation in the process of flow (from the data on lava fluxes of the volcano Etna).
We were the first to measure the BSD in products of the experimental degassing of water-containing melts at a gradual decompression. It has been shown that the BSD of the explosive activity products corresponds to degassing with the predominance of heterogeneous nucleation with a low energy barrier. It has been established for the first time that the experimental BSD, in total, are fairly described by the power distribution with the exponent different from the one characteristic of lava fluxes.
In our opinion the work contains new and important results. So far, the literature reports only rough estimations of the BSD of rhyolite glass samples heaved at a drastic decompression (see in the work of Lyakhovsky et al., 1996). Cashman and Mangan (1994, 1996) are making persistent attempts to use the exponential representation of the BSD of natural degassed glasses based on the model of the open system wherefrom bubbles escape with the probability associated with their sizes and the time period of their stay in the system. The similarity of the BSD of the samples which we obtained in practically closed system with the natural ones (Mt. Etna - our work; Mangan and Cashman, 1996) suggests that their interpretation is erroneous. We claim that we have created the base for the experimental work and theoretical analysis of the BSD evolution in time.
# Litvin Yu.A. Boundary reactions of active plumes and high-pressure experiment.
key words [plume mantle boundary reactions alkaline rocks]
The present-day yet scarce geochemical and petrochemical data are compatible with the idea of chemical activation of the mantle plumes fronts followed by the formation of alkaline-fluid melts enriched in alkali, fluid, incoherent, and rare elements. The chemically active high-temperature agents of the plumes may, by accomplishing boundary reactions with surrounding rocks, give rise to parental melts for alkaline rocks of intraplate series. The problem of genesis of alkaline basalts in oceanic islands like of continental alkaline series including diamond-bearing kimberlites and lamproites are of crucial importance for geodynamic constructions. These schemes have, however, no clear physicochemical and experimental substantiation. The problems posed to a high-pressure experiment stem out of the necessity of knowing specific features of boundary reactions of the chemically active agents of the plumes with ultrabasic substance of the host mantle.
The problem of the chemical composition of the active agent at the mantle plume front is most important. The data on inclusions of alkaline melts in the mantle rocks and minerals can be used to advantage in modelling this composition. The data are listed in the table.
|
Oxides |
1 |
2 |
3 |
4 |
5 |
|
SiO2 |
63.20 |
64.20 |
61.30 |
64.90 |
54.30-66.00 |
|
TiO2 |
0.95 |
0.68 |
2.10 |
0.40 |
0.10-2.20 |
|
Al2O3 |
16.57 |
16.94 |
14.50 |
17.20 |
15.60-22.40 |
|
FeO |
3.58 |
1.60 |
0.40 |
1.00 |
0.70-5.00 |
|
MgO |
2.82 |
1.54 |
- |
- |
0.60-4.10 |
|
CaO |
1.74 |
1.02 |
0.06 |
- |
1.40-11.00 |
|
Na2O |
4.17 |
5.44 |
2.10 |
0.20 |
2.20-7.40 |
|
K2O |
6.26 |
8.39 |
8.50 |
16.70 |
1.00-6.90 |
|
CO2 |
supersaturated |
||||
|
H2O |
over 1.20 |
||||
|
LREE |
enriched |
Note: 1-inclusions in 21 mantle olivines; 2 - intergrain melts in 14 mantle peridotites; 3-central inclusion in diamond; 4 - scattered inclusion in the yellow diamond shell; 5 - melt inclusions in mantle peridotites from continental and oceanic intraplate provinces (Schiano et al, 1994); there are also silicate-carbonate and carbonate inclusions.
The model compositions of the chemically active agents of the mantle plumes are quite complex, multi-component, variable, and exhibit an increased alkalinity. The simplifications required in physicochemical experiment are achieved by using model Na-alkali alumosilicate components, e.g. NaAlSi2O6 (jadeite) and NaAlSiO4(nepheline) and K-alkali carbonate components, e.g.
# The work has been supported by Russian Foundation for Basik Research project N 96-05-64786
8
K2CO3. The choice of the systems of components for experimental high-pressure studies is also motivated by the fact that the main with respect to the concentration, component of the ultrabasic mantle is Mg2SiO4 (forsterite). For example, the mineral composition of the upper mantle is estimated to be
[Ol(Fo,Fa)]60[Opx(En,Fs)]20[Cpx(Di,Hd,Jd)]10[Ga(Py,Alm,Gros)10] (wt%).
Our high-pressure experiments concerned with a study of the boundary interaction between the mantle plumes and the host ultrabasite lithosphere reveal a very high reactivity of the high-temperature chemically active agents of the plumes with respect to the main components of the host mantle.
The investigations of the Na-alkaline silicate system forsterite-nepheline-silica have yielded the following results:
-in the subsolidus of the binary join forsterite -jadeite above 4.5 GPa the reaction Fo+Jd = Py + (Jd, En)ss+ Na2Mg2Si2O7 takes place; thereupon formed Na-Mg-silicate (NMS7) is a new subject of the experimental mineralogy and is, possibly, a mantle mineral (Litvin, Gasparik, 1995, Gasparik, Litvin, 1997).
- in the subsolidus of the other binary join enstatite -nepheline above 4 GPa the reaction Fo+Ne=Py + NMS7 proceeds (Gasparik, Litvin, 1997 );
- the low-pressure phase-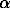 -NMS7 above 3.6. GPa exhibits the polymorphic transition to the high-pressure phase -
-NMS7 above 3.6. GPa exhibits the polymorphic transition to the high-pressure phase -  -NMS7 (Litvin, Chichagov, Bondarenko, 1997);
-NMS7 (Litvin, Chichagov, Bondarenko, 1997);
- the PT-melting curve of NMS7 has been studied to 22 GPa; it has been found that this is the most fusible mantle component (Gasparik, Litvin, 1997);
- it has been found that the meteorite mineral Na-roederite Na2Mg5Si12O30 and other Na-Mg-silicates Na2MgSiO4, Na4Mg2Si3O10, Na2Mg2Si6O15 are unstable under high pressures, and disproportionate within 3-6 GPa yielding simpler compounds (Litvin, 1997);
- the system forsterite-jadeite investigated at 6 GPa demonstrates pseudobinary phase relations and is characterized by peritectic point on the solidus (Gasparik, Litvin, 1997 ).
The experimental studies of the K-alkaline silicate -carbonate system forsterite-K2CO3 under high pressures have made it possible to establish:
- the reaction Fo+K2CO3 = MgCO3 + K2SiO3 + MgO at 3.7 GPa;
- the reaction Fo+K2CO3 = K2Mg(CO3)2 + K2SiO3 + MgO at 3.7 GPa;
- the pseudobinary character of the system Fo -K2CO3 as the join of the ternary system Fo - K2CO3 - K2SiO3, herewith the phase equilibria of the solidus are characterised by three eutectic points;
- the compound K2Mg(CO3)2 was employed as the base of the alkaline-carbonate melt which effected recrystallization of graphite to diamond crystals with their spontaneous nucleation at 9-11 GPa (Litvin, Chudinovskikh, Zharikov , 1997).
The obtained experimental results are important for the problems of generation of primary alkaline mantle magmas, origin of alkaline basalts in oceanic islands, ultrabasite-basite differentiation of mantle magmas, nature of 400 km seismic boundary, origin of primary magmas of intraplate continental series, physicochemical conditions of diamond genesis.
Salova T.P., Osadchii E. G., Epelbaum M.B. Experimental determination of pO in Ab-Q and Na2O-SiO2 melts.
key words [basicity melt experiment]
Relative basicity of melts: Ab with respect to eutectic Ab-Q and Na2O× 2SiO2 with respect to Na2Ox3SiO2 has been measured using precision apparatus. Relative basicity of the Ab with respect to the Ab-Q was measured with a traditional galvanic cell (using a solid electrolyte) where PO2 in the common gas space could be preset in broad limits. The cell design made it possible to change the gas medium in the course of the run (blowing out). Commercial argon containing approximately 104 %O2 and air (20.96% O2) were used as the gases. The oxygen pressure in the cells was controlled with a cell with the Ni/NiO buffer isolated from the gas space by the eutectic melt Ab-Q. The temperature was maintained with an accuracy of +0.5K, the EMF was measured with an accuracy of +0.01 mV. The T and EMF measurement were performed under the automatic conditions with the preset time interval (normally 10-600s), and were represented in the form of a digital or graphic file by means of a special computer program. So, it was always possible to observe the process kinetics and to fix the equilibrium states. Such possibility was particularly useful changing the gas in the system. The equilibrium restored in a period of 9 to 15 h.
The results of the measurements of dpO in the system after the transition to a steady state at different T and in different gas media attest that the measurements in the units with the common gas medium are identical. It has been shown that the composition of the Ab-Q eutectic are more acid than the albite melt by approximately 0.04, 0.12, 0.18 dpO units at temperatures 1485, 1439, and
9
1400K, respectively.
The melts of the system Na2O-SiO2 (with the ratio 1:2 and 1:3) were studied using a cell with a solid PO2 buffer (Ni/NiO2). The inner volume of the cell was prevacuated, then the O2 pressure was set corresponding to the buffer employed. The EMF measurements were performed between the common gas electron and the electrodes of the melts. EDS 1 and 2 were measured individually. The third, auxiliary, cell (Pt, PO2, (Ni/NiO)/YSZ/ PO2,(gas), Pt) where YSZ was Y2O3-stabilized ceramics (9 wt%) was intended for the gas medium control. Our dpO results are comparable with the ones reported by V.G.Konakov [1]. We believe that the constructive development of such an element of comparison is a prospective task to perform. Should it make a success an all-purpose high-stability electrode can be produced.
Fig.1 gives temperature dependencies obtained in the afore described two run series.  E is seen to decrease with temperature, this increase being considerable. Such a course is quite clear since the degree of the melt dissociation grows with the temperature.
E is seen to decrease with temperature, this increase being considerable. Such a course is quite clear since the degree of the melt dissociation grows with the temperature.
References:
# Chevychelov V.Yu. Solubility of chlorine in calcium-containing granitoid melts. Correlations in the melt composition .
key words [solubility calcium melts compositions]
New experimental data on the solubility of chlorine in the SiO2-Al2O3-CaO-Na2O-K2O melts with the granodiorite, granite and leucogranite compositions for the starting 1m NaCl + 0.1m HCl solution at T = 800-1000oC and P = 1 and 5 kbar are presented. The value of this solubility increases as the temperature increases and the pressure decreases (Fig.1) for all of the three compositions studied. The maximum content of chlorine of ~0.8-0.9 wt.% was obtained in the presence of the granodiorite melt at P = 1 kbar and T = 1000oC. We relate this elevated content, along with the comparatively high temperature and low pressure, mainly to the effect of the chemical composition of the melt, in particular, to the content of Ca and Si. Using the published data on the solubility of chlorine in melts with the basalt, andesite (Gorbachev and Khodorevskaya, 1995) and phonolite (Metrich and Rutherford, 1992) compositions, we estimated the significance of the correlations between the chlorine content in the melt and the concentrations of the major elements and their molar ratios. It is shown that a positive correlation with the confidence probability 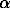 =0.99 is observed between the content of Cl and CaO (Fig. 2).
=0.99 is observed between the content of Cl and CaO (Fig. 2).
The effect of the melt composition on the solubility of chlorine is related to several major elements. In addition to sodium and potassium, an elevated content of calcium, perhaps against the background of a reduced content of silica, is very significant for the compositions studied. The most significant positive correlations in a wide range of compositions (from tholeiite and phonolite to leucogranite) are observed between chlorine and the molar ratios of CaO/SiO2, CaO/Al2O3, and Ca in the melt composition. This correlation is likely caused by the formation of mixed oxygen-halide anionic groups around Ca2+ cations in the silicate-salt melt (Anfilogov et al., 1990), which results in the formation of a unified system of bonds between "autocomplexes" (of the CaCl+ type and perhaps more complicated CaCl42-, CaCl64-) and silicate anions and prevents the combination of halide and silicate anions to separated groups preceding lamination of the melt.
Fig.1. Dependence of the chlorine content in the melt on silica content at different P-T parameters.
Fig. 2. Dependence of the chlorine content on the CaO content in the melt at P = 1 and 5 kbar. Our data are shown by black squares and circles; published data (Gorbachev and Khodorevskaya, 1995; Metrich and Rutherford, 1992) are shown by white squares and circles. Lines correspond to linear regression equations.
In the composition range studied, we obtained significant negative correlations between Cl and the molar Na2O+K2O/Al2O3, which is the agpaitic coefficient (Ka). This coefficient is the most universal parameter, which controls the solubility of chlorine in both calcium-free and
# This work was financially supported by the Russian Foundation for Basic Research (Grant 96-05-64709), RFBR-DFG (Grant 96-05-00020G), and Internationla Science Foundation (Grants MUR000 and MUR300).
10
calcium-containing melts (Fig.3). The content of chlorine increases as the agpaitic coefficient decreases for the studied compositions of the subalkaline and normal series (at Ka<1), as this coefficient increases for the alkaline compositions (at Ka > 1), and it is minimum at Ka = 1.
Fig.3. Dependence of the chlorine content on the molar Na2O+K2O/Al2O3 in the melt composition. The data by Metrich and Rutherford (1992) are shown by triangles and oblique crosses: P=1 kbar, T=850oC. Straight crosses show the data by Webster (1992): P=2 kbar, T=800oC, 4 wt.% Cl in the fluid. For other designations, see Fig.2.
It is shown that in the range from the leucogranite to at least tholeiite compositions the chlorine concentration increases as the content of silica in the melt decreases. This is related to the fact that for the silicate and halide components the mutual solubility decreases as the content of SiO2 increases, and immiscibility in similar melts is a consequence of the incompatibility of the ion structure of typical salts with the ion-covalent structure of SiO2 (Anfilogov et al., 1990).
We suppose that for the Ca-containing aluminosilicate melts, the bonds between Cl and Na and K established for the SiO2-Al2O3-Na2O-K2O compositions (Metrich and Rutherford, 1992; Webster, 1992; Malinin and Kravchuk, 1995) are replaced by predominant bonds between Cl and Ca, and a certain specificity of the Ca-containing aluminosilicate melts toward the solubility of Cl can be discussed.
References:
# Bogolepov M.V. Experimental study of the compositions of the first melt portions at granitization of amphibolite.
key words [fluid melt decompression]
It was proved theoretically (D.S.Korzhinsky, 1973) and experimentally (V.A.Zharikov et al, 1990) that acidic melts can form under the action of a deep fluid an basic rocks. However, the effect of such deep fluids, depending on their compositions, on the composition of first melt portions at granitization of basic rocks has not been experimentally modelled.
The aim of this work was to determine the compositions of the first portions of the forming melts in accordance with the composition of the model deep fluid. To this end the following experiments were performed: 1) at Pfl - const, 2) at fluid decompression.
A charge of the Baikal amphibolite was inserted into the lower capsule part (table 1). A crystal of a mineral-melting indicator was positioned in the upper capsule part. In our case the solution-to-charge ratio was 1:1. H2O and 1n HCl solutions were used. The runs were performed in a hydrothermal high-pressure apparatus provided with an external heating.
Such experiments model the formation of an acidic melt immediately on a quartz absent substrate, i.e., amphibolite, upon decompression of the fluid of different compositions from 6-5 kb down to 4-3 kb. The charged capsule was placed under the experimental conditions.
The capsule was kept under P=6kb and T=750oC for 1 to 10 days. After such duration the pressure was isothermally decreased and the capsule was kept for 1-2 days under a lower pressure. The chemical compositions of the produced melts were measured on an X-ray microprobe Camebax.
It has been found that no melt forms on minerals-indicators in 7-day-long runs without decompression even with high concentration of alkalies and SiO2 in the fluid, although the dissolution of both amphibolite and plagioclase is in progress. The composition of the fluid in equilibrium with amphibolite was determined in the work of L.I.Khodorevskaya and V.A.Zharikov, 1997, the compositions of the solutions in equilibrium with plagioclases were determined by us using the technique designed by T.P.Salova, 1983 (table 1). The review of the data on the incongruent solubility of the mantle substance was given in the paper of V.A.Zharikov et al.,1990. All the works demonstrated high solubility of SiO2 and R2O in the fluid.
# The work has been supported by RFBR grant N 96-05-64710
11
Table 1. Compositions of the starting materials and the experimental results on the determination of the fluid composition in equilibrium with them (wt%) P=5kb, T=700oC
|
Amphibolite |
Labrador |
Olicoclase |
* |
|||
|
Oxides |
start |
fluid* |
start |
fluid** |
start |
fluid |
|
SiO2 |
49.64 |
62.7 |
55.41 |
73.45 |
65.04 |
70.26 |
|
Al2O3 |
16.65 |
1.4 |
27.75 |
15.05 |
21.59 |
16.08 |
|
FeO |
8.88 |
1.8 |
0.22 |
0.01 |
- |
- |
|
MgO |
8.80 |
2.6 |
0.06 |
0.29 |
- |
0.44 |
|
CaO |
9.01 |
2.6 |
10.40 |
0.08 |
1.91 |
0.2 |
|
Na2O |
3.00 |
21.8 |
5.46 |
7.43 |
11.13 |
10.13 |
|
K2O |
1.90 |
4.2 |
0.26 |
3.73 |
0.24 |
2.81 |
Note: * -Khodorevskaya L.I., Zharikov V.A., 1997; ** - our data
Table 2. Compositions of the produced melts in the decompression runs as compared with the starting amphibolite.
|
Olig 5-3 |
H2O 6-4 |
1n HCl 6-4 |
Amf-Balk |
|
|
SiO2 |
71.43 |
71.70 |
76.67 |
49.64 |
|
TiO2 |
0.14 |
0.05 |
0.03 |
1.54 |
|
Al2O3 |
16.84 |
17.29 |
15.56 |
16.65 |
|
Fe2O3 |
- |
-0 |
- |
1.64 |
|
FeO |
0.16 |
- |
0.10 |
8.88 |
|
MnO |
- |
- |
- |
0.15 |
|
MgO |
- |
0.10 |
0.33 |
8.80 |
|
CaO |
0.82 |
1.36 |
2.44 |
9.01 |
|
Na2O |
6.38 |
7.06 |
1.67 |
3.00 |
|
K2O |
4.36 |
2.40 |
3.33 |
1.90 |
|
P2O5 |
- |
- |
- |
0.32 |
|
Cr2O3 |
- |
- |
- |
- |
|
CO2 |
- |
- |
- |
- |
The composition of the formed melt as compared with the starting rock, depending on the fluid composition, is given in table 2. The data clearly show that the concentration of SiO2 grows in the first melt portions upon decompression. From 49 wt% in the starting amphibolite to 72 in H2O and 76 wt% in 1n HCl in the formed melts. The runs with H2O and 1n HCl demonstrate that the difference in the compositions of the formed magmas at amphibolite granitization is due to the fluid composition. These experiments confirm D.S.Korzhinsky's idea of metamagmatic debasification as a transmagmatic fluid alters the magmatic melt composition.
No melting was observed in the experiments run under a constant pressure without decompression. A film of an acidic melt forms on the indicator only at decompression. This can be attributed to the fact that at incongruent dissolution of basic and ultabasic rock minerals the chemical potential of SiO2 does not correspond to the chemical potential of SiO2 of the quartz proper. But at decompression (fluid unloading) there will form an excessive SiO2 , and its chemical potential will be sustained by quartz now rather than by amphibole. This, precisely, causes melting of the minerals-indicators.
So, the decompression experiments with fluids of various compositions suggest that the variability of the granitoid compositions depends on the fluid conditions.
# Suk N.I. Experimental study of the extraction of ore metal (W,Ti,Zr,Tr) by chloride salts.
key words [melt metals extraction experiment]
The systems of alumosilicate melts with admixtures of ore components (Na2WO4, TiO2, ZrO2, La2O3, CeO2) and NaCl salt have been studied experimentally in a high gas pressure apparatus, in sealed platinum capsules at T=1250oC, P=2kb.
Fig. Schematic presentation of chloride extraction of tungsten from silicate melts on the diagram of their standard mineral composition (from the experimental data).
1-initial compositions of the experimental samples. 2-compositions of silicate glasses after the separation of the chloride melts; 3-standard compositions of minerals: Ab-albite; And-andalusite; Di-diopside; Q-quartz; Cor-corundum; Ne-nepheline.
The experiments have systematically shown that the initial melts got separated into two liquids, namely, sili-
# This work has been supported by the Russian Foundation for Basic Research, project N 97-05-64158
12
cate liquid and salt (chloride) liquid which showed up as layers with a clear phase boundary between the melts.
As contrasted from phosphorus which is closer associated with the alumosilicate layering of alkaline intrusions and which concentrates in their horizons rather depleted in silica, (urtite horizons), the behavior of chlorine more closely characterizes the migration of oregenous metals from them. This is accounted for by the more ready solubility of chlorides in aqueous solutions as compared with phosphorus salts, and by weaker bonds of chlorine with aluminosilicate melts against those of phosphorus. In those characteristics chlorine belongs to magmaphobic components whereas phosphorus exhibits clear magmaphilic properties.
In the experimental studies this difference in the elements is manifested in the specific features of separation of the salt phases from the aluminosilicate melts. In the chlorine system purely saline melt phases (NaCl) separate and selectively accumulate oregeneous metals whereas phosphorus melts are, normally, complex in the composition and enriched in oregeneous and petrogeneous metals.
A very high efficiency of the chloride extraction of oregeneous metals from aluminosilicate melt was demonstrated by us with tungsten. Tungsten practically completely passes into a salt melt NaCl which segregates from the initial tungsten melt and forms in it isolations containing to 4-4.5 at.% W (22-24 wt% WO3), and, with calcium available, it forms isometric grains of scheelite containing 0.05-0.03 wt% Na2O. The efficiency of chloride melts with respect to tungsten extraction is explained by the formation in them of chloride complexes with tungsten, for example, Na2[WCl8] -Na2[WCl6O] -Na2[WCl4O2] - Na2[WCl2O3], fig.1.
The chloride extraction of metals, like any other salt extraction, has a selective character. This was observed by us upon introducing titanium and zirconium into the studied system. As contrasted from tungsten, these metals are not virtually extracted from aluminosilicate melts by chloride melts. The experiments have shown that the silicate melt differs from the initial compositions in an increased concentrations of Ce, whereas the behavior of La is ambiguous. In the albite-salt systems La also demonstrates increased concentrations in a silicate melt; in diopside -containing systems the silicate phase is depleted in this element as compared with the initial compositions, and within the salt phase (NaCl) there are Ca-,Mg-, La-, and Ce-rich inclusions (possibly, chloride compounds of said elements). So, additional studies still have some way to go.
In fact, the chloride extraction of metals is an important stage in the migration of orogeneous metals from magmatic chambers. This stage determines the evolution of ore deposits distant from magmatic chambers which generate them.
#
Shchekina T.I., Gramenitsky E.N. Behaviour of trace elements in felsic fluorinebearing magmas depending on their composition according to experimental data.key words [melt salt distribution ore metals fluorine granite]
This work deals with the ore components concentration mechanism at the magmatic stage. It is presumed that salt or silicate-salt melts necessarily appear during the course of differentiation in the residual melts as a result of their saturation by salt and volatile components. We suppose that salt melts, which are called fluid melts in this work, effectively extract ore components. The model is based on our experimental data (Geohimia, 1993) in the system Q-Ab-Or-H2O-F in which there is great field of liquid immiscibility between aluminosilicate and alkalialuminofluorine melts in the liquidus and subliquidus regions. The ranges of aluminosilicate melts compositions, considered in our experiments, cover both agpaitic (potassium-, potassium-sodium, sodium-and lithium-rich granites) and peraluminous ones.
This paper presents the results of the experimental data on partitioning of a wide range of trace elements (Li,Nb,Ta,Zr,Hf,W,Mo,Pb,Zn,Sc,Th,Y,REE) among silicate and salt melts coexisting in granite system with fluorine.
All experiments were performed at the same temperature of 8000C and at the pressure of 1 kbar with the help of a hydrothermal apparatus (of Tuttle's bomb type) during 3-4 days. In most cases the metals were introduced to the charge as oxides in the amount of 1-2 wt%. The content of water was 4 wt.%. The trace element content in the phases was determined by microprobe analyses with the help of Camebax-MBX.
The two-liquid partition coefficients (here defined as KD=metal content in silicate melt, wt.%/ metal content in aluminofluorine melt, wt.%) have shown complicated dependencies on the system composition. It is shown that the coefficients dependence on K/(K+Na) ratio in the granitic system. W,Sc and Li are concentrated in the aluminofluorine (fluid-) melt, the other elements- in the silicate one, but with different partition coefficients. When K-content of the system increases, the most of trace elements (Hf,Nb,Ga,Ge,Zn,Th,Sc,REE,W) tend to decrease their partition coefficients. But Pb and Ta coefficients increase in this case. It is noted that partition coefficients and their variations of Nb and LREE, also Th and HREE are close to each other. Different variations of partition coefficients of chemically close elements are correlated with the changes of the system composition and explain the differences of 'geochemical indicators` values, such as Ta/Nb, Zr/Hf, LREE/HREE. These relations are presented as a ratio of suitable partition coefficients among alumosilicate and aluminofluorine melts (KDNb/KDTa/, KDZr/KDHf, KDLREE/KDHREE). Potassium content in the sys-
# This work is supported by the Russian Foundation for Basic Research
13
tem increases ratios Zr/Hf and LREE/HREE, but decreases Nb/Ta. These trends correlate with natural observations.
Partitioning data for trace elements in peraluminous part of the system permit us to divide all elements into two groups. KD of the first one (Ta,Nb,Mo) decrease as agpaitic coefficient increases; KD of the second elements group of (Hf, Zr,Ga,Th,REE,W, Sc) tend to decrease in peraluminous area of the system, that is the silicate melt is depleted in the second group elements. W and Sc are distributed in favour of aluminofluorine melt in this case. Values of `geochemical indicators` Ta/Nb, Zr/Hf, LREE/HREE increase when moving from peraluminous area to the agpaitic part of the system.
The Li introduction into the system strongly changes the partition coefficients of many elements: all REE,Y,Pb,W are taking coefficients in favour of fluid melt. The contents of Nb,Zr,Hf in the two coexisting melts become similar and their associated partition coefficients approach 1. Hence the lithium existence in the system causes an increase in solubility of the most trace elements in the salt melt. But the strongest effect occurs in REE distribution. It can be explained by a tendency of REE to from complexes with alkali metals and fluorine of (Li,K,Na)3LnF6 type, which naturally concentrate in aluminofluorine melt.
Conclusions
1. The present experimental investigation of ore elements partitioning among the coexisting silicate and aluminofluorine melts proves that fluid melt effectively concentrates many ore elements. It gives additional and sometimes the only possibility of the elements concentration even at the magmatic stage and is the only mechanism which explains the available data for the association of ore deposits with granite intrusions.
2. The above mentioned features are not universal ones. The elements fractionation among the coexisting melts is of a selective character and depends on the system composition.
3. It has been found out that the tendency to concentrate the elements with closely related properties (such as Ta-Nb, Zr-Hf, Pb-Zn, Mo-W, LREE-HREE) is different in relation to the system composition. These data, in particular the oppositely directed tendencies in the behaviour of Ta and Nb, Zr and Hf, LREE and HREE explain the changes of geochemical indicators (Nb/Ta, Zr/Hf, L/HREE) in different types of granites.
Kolonin G.R., Sinyakova E.F., Peregoedova A.V. Mineral forms of platinum group elements (PGE) as reflection of physical-chemical evolution during fractional crystallization of Cu-Fe-Ni-S sulphide melts (experimental data).
key words [sulphide melt platinum group elements]
The wide experimental study of the features of crystallization of sulphide melts has been carried out with starting charge compositions corresponding to the Me9S8 section of Cu-Fe-Ni-S system and to some important sections of Fe-Ni-S system, always with small additions (0.2-0.4 at .%) of Pt, Pd, Rh, Ru, Ir (Kolonin et al., 1993, 1994, 1997; Sinyakova et al., 1994, 1996, 1997; Fedorova et al. 1996). Experiments were carried out at several temperatures within the interval of 11000-4000C. Sulfur fugacity was measured in these runs at 900, 760, 550 and 4000C by the pyrrhotite method (Barton, 1964). The chemical composition of different phases were determined using Camebax microprobe.
Table. Temperature dependence of main mineral forms of PGE.
Cu | Ni | Fe | S | Fe | Ni | S |
|
toC |
Pt |
Pd |
Rh |
Ru |
Ir |
toC |
|
870 |
PtFe,Pt3Fe melt (<0.1Pt) |
melt (up to 0.8 % Pd) |
- |
- |
- |
|
|
760 |
Pt(Fe,Ni) with 1.6%Pd Pt3(Fe,Ni) up to 2.2%Pd PtS up to 4.5%Pd |
Mss up to 1.1%Pd Cu-Hzss to 1.1 %Pd Ni-Iss to 0.9%Pd vysotskite 0.410%Pd |
Mss to 0.8 Rh Hzss to 0.8%Rh |
Mss to 1.2 %Ru Hzss to 0.3 %Ru
|
intermetallic phase Ir0.6-0.7Fe0.4-0.2Ni0.1 |
800 |
|
550 |
Pt(Fe,Ni) Ni to 12% Pt3(Fe,Ni,Cu) Cu 2 (Pt,Ni)S Ni up to 6% Cu(Pt,Fe)2S4
|
Ni-Mss (to 0.2%Pd) Fe-Pn(to 0.4%) or
vaesite (to 0.3%) |
Mss (to 1% Rh) Pn (to 0.3%) or
CuRh2S4 (Rh,Ni,Cu)S2 |
Mss (up to 0.4% Ru) Fe-Pn(up to 0.4%) or |
Mss (up to 0.2% Ir) Pn (up to 0.2% Ir) |
600 |
|
400 |
Pt(Fe,Ni) with 0.8% Pd and rims (Cu,Fe)Pd Pt3Fe with 1% Pd and Pn with 1%Pd (Pt,Pd,Ni,Cu)9S8 together with Cu-Pn (up to 1% Pd) braggite (Pt,Pd,Ni)S Cu(Pt,Ni)2S4 |
- |
- |
|||
14
Important experimental results were obtained, describing the behavior of Pt, Ir and light PGE (Pd,Ph,Ru) at fractional crystallization of sulphide melts. The principle role of pentlandite was established after its appearance at temperatures from 6130C (in the system without Cu) to 574 (in Cu-rich systems) as a bearer of light PGE, when Pt and Ir stood out as a separate intermetallic forms.
Three stages of formation of PGE-containing phase assemblages were distinguished conventionally: high-temperature pre-pentlandite (1100-600oC), middle post-pentlandite (580-400oC) and low-temperature ones (the last stage was behind of the present study yet). At this time the physical-chemical conditions of stability of different PGE mineral forms were graduated in dependence from both main parameters, namely temperature and S2-fugacity. The inevitability of essential step-by-step changes of PGE mineral forms during fractional crystallization of sulphide melt has been shown too. The most important information about mineral forms of PGE is presented in the Table. The PGE-partition coefficients between base metal minerals were determined in some cases, using the results of electron microprobe analyses. The perfect correspondence of sulfur fugacity to the ratio of base metals was established when fS2 rises under isothermal conditions along the increase of (Cu+Ni)/Fe ratio. Such approach allows to elucidate the clear dependence of PGE mineral forms on base metal assemblages in experiments and to suppose the same picture under the natural conditions. A conclusion about the importance of elucidated regularities related to dominant PGE phases for improvement of technologies of their extraction from sulphide ores is emphasized.
Smolkin V.F. The Pechenga ore field: Liquid immiscibility on ferropicritic rocks.
key words [ferropicritic rocks immiscibility sulphide]
Unique volcanic associations have been distinguished and examined in the Pechenga region. The association includes volcanic ferropicrite rocks occurring in flows of massive and pillow lavas, bedded sill-like bodies and seams of lava-breccias and tuffs. They are composed of the same mineral paragenesis (olivitne-chrysotile+titan-augite + kaersutite plagioclase-labradorite + titanochromite + ilmenite), have similar isotope characteristics (T = 1980+40 Ma, 143Nd/144Nd = 0.510148; 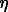 Nd = +1.5; 87Sr/86Sr = 0.7032; 232Th/236U = 3.8-3.2; 186Os/188Os = 935 + 0.031) and geochemical features (increased contents of TiO2, Fe2O3 + FeO, Ni, S, P2O2), but they differ in the degree of differentiation and ore potential (Hanski et al. 1990; Smolkin 1992; Walker et al. 1994).
Nd = +1.5; 87Sr/86Sr = 0.7032; 232Th/236U = 3.8-3.2; 186Os/188Os = 935 + 0.031) and geochemical features (increased contents of TiO2, Fe2O3 + FeO, Ni, S, P2O2), but they differ in the degree of differentiation and ore potential (Hanski et al. 1990; Smolkin 1992; Walker et al. 1994).
Several models have been proposed to explain the genesis of the sulfide ores; among them the liquid immiscibility-magmatic model is the most widely accepted. Until now, the evidence for liquid immiscibility of silicate melts or a silicate and a sulfide melt has been based on experimental data, rather than geological observations. Various reasons can be responsible for this, including the effects of later magmatic crystallization, extensive metasomatism due to the abundance of volatiles in the ore melt, and a considerable alteration of rocks and ores that took place during folding and regional metamorphism (1750-1700 Ma).
A key to decipher the conditions of differentiation of Ni-bearing ultrabasic melts is provided by layered flows. Layered flows are common in the thick volcanic Matert Formation, Pechenga and occur in three levels, being alternated with tholeiite-basalt lavas and tuffs. The thickness of the flows varies from 3-5 to 50 m; some of them have been traced over a distance of 2.5-3.0 km. The most differentiated flows are composed of (from bottom to top) the lower chilled zone, olivine cumulate zone, clinopyroxene cumulate zone, globular rocks zone, a zone with a spinifex-texture of the olivine or pyroxene type, and finally, the upper chilled zone. Less differentiated flows consist of the spinifex-texture zone followed by globular rocks. Globular rocks also occur in sill-like bedded bodies. The globules are represented by pale-grey ball-shaped fine-crystalline aggregates 2-5 to 25-30 mm in size, rarely 50 mm. They have a sharp boundary with the darker matrix, and in places the boundary is marked with a light-coloured fine-grained rim. Apart from silicate globules, scarce flattened sulfide balls composed of pyrrhotine or pyrrhotine and chalcopyrite are present. First suggestions about the origin of the above textures as products of liquid immiscibility were published in (Smolkin et al. 1987).
The following observations may serve as evidence of liquid immiscibility:
1. Morphological. In natural outcrops one can note all stages of globule origin, convergence, caving-in and agglutination, which in some cases proceed up to the formation of pseudolayers with the preserved inter-ball matrix. The lower boundary of these pseudolaers is sharp and sinuous; the upper boundary is more complicated due to gradual decrease in the number and the size of globules. Small globules in places were displaced to the upper surface of the flow, apparently, as a result of flotation.
2. Mineralogical. The globules and the matrix are composed of the same, originally magmatic paragenesis (titanaugite, kaersutite, ilmenite, sphene, and relics of albitechloritized glass), although the matrix contains more titanaugite and less glass than the globules. The composition of the cement in the globules and the matrix is different due to the different ratio of orthoclase to plagioclase phases (the matrix normally is rich in orthoclase). Titanaugite in the globules commonly forms sheath-like crystals, which near the globule boundaries become split and form 'bird's-tail` textures. An analysis of complex, zoned titanaugite grains from the globules and the matrix showed that their cores are similar in composition, while the inner and outer zones differ in the TiO2 content and the FeO/(FeO+MgO) ratio.
3. Geochemical. The zone of globular rocks is strongly enriched in TiO2 and has elevated contents of volatiles (P2O5, F, S). While the FeO/(FeO+MgO) ratio is relatively constant, the SiO2 and alkali contents and the Na/K ratio in the globules and the matrix are different, the globules being enriched in SiO2 and Na, and the ma-
15
trix in K. In addition, the globules and the matrix differ in REE, Sr, Rb, Zr, Sc, Ta, and Ba contents.
4. Isotope-geochemical. Re-Os results indicated that the globules have a very high 186Os/188Os ratio. This fact is difficult to explain in terms of crystal differentiation, but is easy to confirm by experimental studies of liquid immiscibility.
All the above data suggest that the process of liquid immiscibility proceeded in several stages and resulted in the formation of not two, but three immiscible melts. The process started after the crystallization of olivine and earlier clinopyroxene phases was completed, and was almost instantaneous. The necessary conditions for the liquid immiscibility in the studied melts are: (a) subaqueous eruption; (b) a considerable thickness of lava flows (over 8-10 m); (c) the formation of the upper chilled zone, and (or) very rapidly spread overlying lava flows with the next portions of lava or turbidity sediments; (d) high-Fe composition of the melt and the initially increased content of volatiles that are elements-modificators (P,F); (g) accumulation of volatiles in a limited volume of the flows (in their upper part), which causes an abrupt rise of their concentration and provides for the structural rearrangement of the melt as a result of broken Si-O bonds.
References:
Kravchuk I.F. , Malinin S.D. , Dernov-Pegarev V.F. Fractionation of tungsten in the acidic fluid-magmatic system.
key words [tungsten fluid acidic melt]
Vernadsky Institute of Geochemistry and Analytical Chemistry, RAS Moscow
The results of the works concerned with the determination of coefficients of partitioning of W between the fluid phase and the coexisting with it silicate melt are extremely controversial. These early studies showed the occurrence of a number of experimental difficulties of which one should primarily mention the problem of attainment of equilibrium concentrations of metal in the melt or vapor phases, the problem of reliable control of the atmosphere in the runs, and some others.
The principle specific goals were:
1. To study the partitioning of W taking as an example the simplest model system Ab-Qz in equilibrium with a 1m NaCl solution at T=800oC and P=2 kb wittingly conforming to the homogeneous fluid state. It was necessary herewith to solve a methodic question principally important for an investigation of the elemental partitioning, viz., what is the criterion of the system's equilibrium? For this, as contrasted from other studies, it was necessary to study the partitioning of W by introducing the element separately into the melt and the fluid phases.
2. To reveal the role played by high chlorine concentrations in partitioning of W. For this it was necessary to study the partitioning of W for a broad range of NaCl concentrations: up to 100% ('dry` system Ab-Qz-NaCl).
3. To estimate the coefficient of partitioning of W between two fluid phases KW2=Xfl1/Xfl2 (Xfl1 and Xfl2 being the concentration of tungsten in essentially saline and essentially water fluid phase, respectively) at the parameters of fluid heterogenization : 800oC and 1 kb.
The runs were performed by two different techniques.
1. The greater part of the runs were performed in a high-pressure device with external heating and effective isobaric system of quenching. The redox conditions in the runs corresponded to the Ni-NiO buffer. After quenching the capsules were opened and the phase separation was performed. The complete X-ray spectral microanalysis of glasses was performed in a Camebax Microbeam (analyst V.G.Senin) and, also, tungsten in the solution was determined by the inductively-bound plasma method.
2. The runs in dry systems were performed in a muffle furnace (the temperature gradient did not exceed 10 degrees) at T=1200oC by a method of sand gate in alundum crucibles with inner platinum capsules. After the run the melted columns were encapsulated in epoxy resin and carefully ground. All the four samples exhibited a clear interface between the liquating immicibility and silicate phases. Next, the concentration of tungsten was determined by means of X-ray spectral analysis using scanning near the interface.
Results.
1. The equilibrium value of the coefficient of partitioning of W between the fluid (1m NaCl) and the model melt of the simplified granite composition at 800oC and 2 kb was first obtained.
2. The system approaches the equilibrium at different rate depending on the direction of the process: the equilibrium is achieved faster from 'the bottom` than from the 'top`.
3. The values of the partitioning coefficients increase monotonously with the concentration of NaCl up to the dry salt state. The Kw value for the dry system at 1200oC and 1 bar is 30. In the dry systems (with albite and natural granite) tungsten also transits from the silicate to the saline phase in the case of NaCl and, on the contrary, it stays in silicate at the interaction with NaF which is indicative of a very important difference of these fluids with respect to tungsten.
16
4. Under the conditions of layered fluid the coefficient of partitioning of tungsten between two fluid phases (essentially saline and essentially water) was determined to be 15 (800oC and 1 kb).
In the aggregate, the revealed dependencies of the coefficient of partitioning of tungsten show a principal possibility of formation of an ore bearing fluid at the interaction of chloride solutions with magmatic melts, the fluid layering leading to an additional enrichment in tungsten of its more concentrated phase. Precisely this phenomenon is likely responsible for the high concentrations of chlorides in inclusions of minerals occurring in association with tungsten minerals in the corresponding ore deposits.
#Kravchuk I.F. , Malinin S.D., Slutskii A.B. Behaviour of chlorine in the fluid-magmatic systems.
key words [melt fluid chlorine partitioning]
Vernadsky Institute of geochemistry and Analytical Chemistry, Moscow
A most important part played by volatile components in magmatic, metamorphic, and hydrothermal processes is well-established. Of the other volatiles, chlorine has a special place because for the largest number of elements the presence of chlorine is the main condition for their extraction by water fluids from magmatic melts due to complex formation. The geochemical data on the chlorine concentration in the continental crust are much versatile, this being much lower against the chlorine concentrations in melts yielded from thermobarogeochemical studies. This fact is due to three principal factors, viz, losses upon crystallization, chlorine losses upon magma eruption onto the Earth's surface, losses upon the interaction with fluids. Taking into account the impossibility of quantitative estimation of chlorine losses in the process of evolution of the fluid-magmatic systems and hence the impossibility of estimating original chlorine concentrations in magmas of various compositions, the experimental modelling of the fluid-melt equilibrium seems most correct.
The experimental studies of chlorine solubility in melts of various compositions and its partitioning between the phases were conducted in high-pressure cells of three types, viz., horizontal two-chamber bomb, gas bomb, and piston-cylinder apparatus at temperature 800-1200oC and pressures to 15 kb. Natural and model systems of from basaltic to granitic compositions were studied in equilibrium with water-chloride fluids of various concentrations.
In this work we have summarised our own experimental and published data on chlorine solubility in melts of various compositions. Maximum chlorine concentrations were found in basalts and granodiorites (1.0-1.3 wt%) at a pressure of 2 kb and experimental temperatures 1200 and 1000oC, respectively. As the pressure was increased to 4 kb the solubility of chlorine in the melts decreased by 1.5-2 times. In the acidic melt-1m NaCl equilibria the solubility of chlorine at 800-900oC and 1.5 kbar grows in the series Ab, Ab-Qz, Ab-Ort-Qz, Ab-Ne, natural granite from 0.08 to 0.33 wt%. We have found positive correlations of chlorine solubility with the concentration in a melt of alkalies, calcium, and iron, and negative correlations with the concentration of silicon and, possibly, magnesium. The solubility of chlorine grows with the fluid concentration, being higher in the systems with HCl-containing fluid. The composition (structure) of the melt largely determines the way by which chlorine dissolves in the melt, and its concentration. This is confirmed by the experimental studies of the system Ab-Qz-1m NaCl with different Na/Al ratio in the melt. The minimal chlorine solubility is observed at the molar Na/Al ratio being unity.
The obtained results suggest the mechanism of the chlorine dissolution both as molecular forms (NaCl, KCl, HCl) and via the complex formation of ionic chlorine with excessive aluminium or sodium contained in the melt. The experimental data have been first obtained for the solubility of chlorine in an alumosilicate melt Ab-Ort-Qz in the pressure range to 16 kb (T=850oC). A complex behaviour has been established for the pressure dependence with a maximal chlorine concentration in the melt at 7-7.5 kb. Possibly, this fact can be explained by the change of the co-ordination number of aluminium (from 4 to 6) in this pressure range which also evidences for the existence of chlorine-aluminium complexes.
The experimental data on the coefficients of partitioning of chlorine between the fluid and the melt depending on various factors suggest the idea of creating a model of the behaviour of chlorine in the magmatic process.
The concentration of chlorine in natural samples is largely determined by saturation of the original magmas with water. If the water-saturation proceeds at early stages then chlorine goes to the fluid and at eruption small amounts of it get to the atmosphere. If the magmas get water-saturated upon eruption and no biotite, apatite, and amphiboles crystallize therewith then chlorine goes to the atmosphere. At the transition of chlorine into the fluid the effectiveness of the extraction of ore and other elements depends on the water/chlorine concentration. At high chlorine in the original melt the first portions of the fluid phase contain little chlorides. If the melt had a small original water concentration and fractionation is proceeding for a long time with the chlorine content of 2000-3000 ppm in the melt the first fluid portions will contain to 50% alkali halogenides which is confirmed by the fluid inclusions data.
## Portnyagin M.V., Ariskin A.A., Sobolev A.V. Fractional crystallization of island-arc
# This work has been supported by the RFBR, grant N 97-05-64881 and 95-05-15068
## This work was supported by the Russian Foundation for Basic Research (Grants No 97-05-65909, 96-05-66014 to MVP and AVS, 96-0564231 to AAA).
17
tholeiites: numerical simulation of the natural liquid line of descent.
key words [basalt crystallization fluid numeric calculation]
The presence of abundant magmatic water is a special feature of subduction related magmas. Its initial concentrations may be as high as 3 wt.% [1] in these magmas that is close to the dissolution limit at the pressures of about 1kbar. Fractionation occuring at the shallow depths will, therefore, readily bring the melts to the saturation in water fluid and to subsequent crystallization at the water saturated conditions. This paper is the first to present the results of numerical simulation of such regime of magma crystallization.
The modeling has been carried out using COMAGMAT-3.0 software [2] in slightly changed version which allowed us to calculate the pathways of equilibrium and fractional crystallization of natural multi-component systems at the water saturated conditions. The maximum concentration of dissolved water in melt at given P-T-X conditions has been calculated after [3]. Water content of undersaturated melts was calculated assuming perfect H2O incompatibility with solid phases. On reaching the dissolution limit of H2O in the melt, its equilibrium content has been reduced by 5 rel.% and calculation was proceeded till the next moment of saturation. The water influence on the liquidus temperatures of minerals has been assumed to be a linear function of water content and equal to (in grad per 1 wt% H2O) 18-20 for olivine (Ol), 20-25 for ortho- and clinopyroxene (Opx,Cpx), 45-50 for plagioclase (Plag) [3].
In order to test accuracy of the model, we compared the phase relationships obtained in experiments [4] and calculated in COMAGMAT for the same high magnesian basalt from Lau Basin (ODP Leg 135) (Fig.1). The results show good agreement between these data sets. In particular, our model reproduces very well expanding of Ol and Cpx and reducing of Plag and Opx stability fields at the elevated water pressures. The temperature of phase apearence is predicted with accuracy of about 10-150C. On the plots MgO-Oxides, the calculated compositional path of residual liquid ('liquid line of descent'-LLD) coincides with experimental data in SiO2, Al2O3, TiO2, Na2O and K2O content. Slight variance demonstrate only FeO and CaO. This variance is probably due to small disproportion of Ol and Cpx on the model cotectic and does not distort significantly calculated LLD.
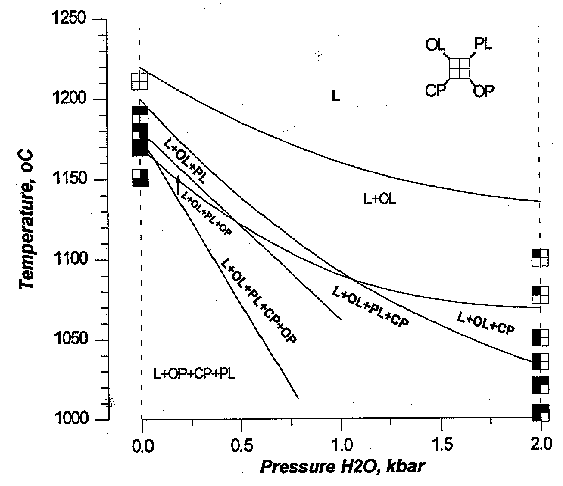
Fig.1. Phase relationships of magnesian basaltic andesite from Lau Basin (DSDP Leg 135) calculated in modified version of COMAGMAT-3.0. Squares show mineral assemblages obtained in experiments [3] at 0,001 and 2 kbar.
The model developed was applied for LLD calculations of island-arc tholeiitic volcanics from northern part of Troodos ophiolite (Cyprus Island). To reconstruct the natural path of magma fractionation, the compositions of 50 volcanic glasses from Akaki River (northern Troodos) canyon were taken [5]. The glasses cover substantial range of compositions from basaltic andesites (SiO2~ 52 wt.%, MgO~ 8 wt.%) to dacites (SiO2~ 62 wt.%, MgO~ 2 wt.%) and were interpreted earlier as cogenetic volcanic suite on the basis of field and geochemical data [5]. Magnesian glass No R316 containing 2 wt.% H2O was choosen as starting point for calculations. Liquidus assemblage of the melt was represented by Ol+Cpx+Plag judging from petrographic observations [5]. All calculations were done assuming crystallization pressure <3 kbar according to the stratigraphic position of the Troodos crustal cumulates.
Fig.2.(a,b)-Calculated paths of fractional crystallization of Troodos lower pillow-lavas: (1) P total=2 kbar, (2)- P total=0.5 kbar, (3) Decompression from 1 kbar following dF/dP=0.01. c.- Histogram of MgO contents in rocks of the andesite-dacitic suite and of the sheeted dyke complex. All oxides are given in wt.%.
18
The following results were obtained. The parental melt could be in equilibrium with Ol+Cpx+Plag assembledge at the pressures 1-3 kbar and water content in the melt~ 2 wt% as it was suggested initially. The equilibrium temperature was estimated to be ~ 11400C and undepedently supported by melt inclusions thermometry in Cpx phenocrysts. The further evolution of the residual liquid is determined mainly by the maximum water concentrations which is capable to be dissolved in the melt at a pressure given in the model. At the total pressure of 2 kbar, the water content increases during fractionation up to 5 wt.%. This results in significant reducing of Plag proportion on cotectic and abrupt deviation of calculated LLD from the natural path in Al2O3 content (Fig.2a). When water content is fixed to be 2 wt.% (water saturated conditions at 0.5 kbar), calculated LLD fits compositions of the glasses much better. At these conditions, typical andesite-dacitic melts can be produced from 50-60% of the parental melt fractionation in the temperature interval of 1140-10500C. Predicted liquidus mineral assemblage of differentiated magmas, Opx+Plag+Cpx+Mt, is in a good agreement with petrographic observations. However, one should note the slope of the natural path. This can result from poor accuracy of the model in low-MgO compositional field. Alternatively, the natural LLD can be well reproduced assuming fractionation accompained by decompression and decreasing water content in differentiates (Fig.2a-b). Such scenario of the crystallization process could take place immediately before the eruption during magma transport to the surface. More magnesian compositions of the Troodos sheeted dykes campared to the volcanics strongly support this suggestion.
In summary, our data show that the natural LLD may be well accounted for by the fractional crystallization of a parental basaltic melt at low water pressure less or equal to 1 kbar. However, the results are in obvious contradiction with direct analyses of the water content in differentiated glasses [5] (Fig.2b) which contain up to 6 wt.% of H2O. The possible resolution of the paradox is that the excess of water in the silica-rich glasses comes from low-temperature alteration followed from the interactions of the quenched glasses with sea water. This is strongly supported in oxigen isotope data reported by [5].
References:
#
Kravchuk I.F. , Malinin S.D. Estimation of thermodynamic properties of sulfur using the experimental data on its partitioning between the fluid and silicate melt phases.key words [melt fluid sulphur activity]
Vernadsky Institute of Geochemistry and Analytical Chemistry, RAS Moscow
Like chlorine, carbon dioxide, and water, sulfur is a basic component of volcanic gases. It actively participated in the formation of the original Earth's atmosphere, and is still of significant effect on the climate. Therefore the estimations of the initial sulfur concentrations in primary magmas and of losses due to eruptions and interactions with water fluids are very important. The geochemical data on sulfur concentration in natural subjects on sulfur concentration in natural subjects are quite diverse whereas practically no direct experimental data exist on its solubility in silicate melts and on its partitioning between fluids and melts. Such studies of sulfur are crucial for the estimation of the unknown thermodynamic properties of fluids involving sulfur.
We report here the experimental results on the partitioning of sulfur between the melt and the fluid in the model system Ab-ORt-Qz-H2SO4 at 850oC and 2 kb in the presence of the Ni-NiO buffer (unpublished data: Kravchuk, Keppler, 1994). The method of estimation of the activity coefficients of sulfur in a fluid earlier developed for chlorine (Malinin, Kravchuk, 1994)) has been used in the work.
The experiments were run in double gold sealed capsules in a high pressure cell with an external heating and the system of quenching to room temperature within 1-2s, with the subsequent separation and analysis of the two phases after the runs. Sulfur was present in the system as solutions of sulfuric acid of various concentrations or was excessively introduced as sublimated sulfur. The table lists the data on the sulfur solubility in the silicate melt and the partitioning coefficients Ds=msmelt/msfluid .
The partitioning of sulfur between the fluid and the melt has the behaviour of a monotonously increasing curve, that signifies the homogeneous state of the fluid over the whole concentration range. Inasmuch as the concentrations of chlorine in the melt are ultimately small (much lower than for sulfur), the activity coefficient of sulfur was estimated by the method of thermodynamic analysis of the two-phase systems (melt-fluid) based on Nernst's law of partitioning and on the assumption of ideality of sulfur dissolution in a melt. Below are given the equations (1-4)) the use of which enables the determination of the activity coefficient of sulfur in the fluid.
Ds=asmelt/asfluid =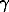 smelt /
smelt /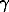 sfluid ×
msmelt/msfluid =
sfluid ×
msmelt/msfluid =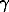 smelt /
smelt /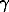 sfluid
sfluid
(1)
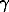 smelt/
smelt/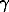 sfluid =Ds/Dso (2)
sfluid =Ds/Dso (2)
Dofl =lim(msdistr/msfluid) (3)
ms 0
0
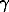 sfluid=Ds/Dso (4)
sfluid=Ds/Dso (4)
# This work has been supported by the RFBR N 97-05-64881
19
|
The fluid composition N H2SO4 |
Sulfur concentration in the melt (wt%) |
Ds |
|
0.10 |
0.004 |
0.026 |
|
0.10 |
0.005 |
0.032 |
|
0.10 |
0.002 |
0.013 |
|
0.25 |
0.007 |
0.017 |
|
0.25 |
0.009 |
0.023 |
|
0.10 |
0.002 |
0.013 |
|
0.25 |
0.007 |
0.017 |
|
0.25 |
0.009 |
0.023 |
|
0.50 |
0.010 |
0.017 |
|
1.00 |
0.013 |
0.008 |
|
1.00 |
0.018 |
0.011 |
|
2.50 |
0.025 |
0.006 |
|
5.00 |
0.049 |
0.006 |
|
H2O+Ssubl |
0.029 |
0.010 |
|
H2O+Ssubl |
0.023 |
0.009 |
The best approximation of the D values for the estimation of ultimate values Dso (linear behaviour of the dependence), like in the case of chlorine partitioning, was obtained in the coordinates logDs-m1/2, in this case the ultimate Dso value is 0.054. The activity coefficient of sulfur in supercritical solutions decreases according to the same law as for NaCl solutions but more dramatically in conformity with lower-temperature dependencies of the behaviour of the activity coefficients of these compounds in water solutions.
Dorfman A.M. Investigation of the viscosity of alumosilicate melts by means of a high - temperature centrifuge.
key words [viscosity alumosilicate melt centrifuge]
Vernadsky GEOKHI;Bayerisches Geoinstitute Universitaet Bayreuth Deutschland
The method has been proposed for the determination of the viscosity of magmatic melts using the falling sphere method with the aid of a high-temperature centrifuge, the gravity being 500-1500 g. The viscosity of standard glass and well prestudied granite melt was employed to demonstrate the validity and accuracy of the method. The viscosity can be determined in the range of 105-108 Pa with the accuracy (based on determination for standard glass DDG-1)+0.09 log units by employing falling platinum balls. This method was also used to study mechanism of iron deposition in a silicate melt.
The melt viscosity of the values higher than accessible for the method of rotating concentric cylinders and lower than accessible for the largest number of dilatometric methods can be obtained by this method. Due to the fact that the viscosity of silicate melts does not obey the Arrhenius law, the data obtained via the centrifuge method of falling sphere used together with the viscosity data obtained by other methods describe much better the temperature dependence of the viscosity than it was possible earlier using the combination of only very high and very low viscosity data. The viscosity-temperature dependences based on the viscosity data of three types yield most accurate estimations of the Tamman-Vogel-Fulcher (TVF) coefficients and exclude the possibilities of breaks in the viscosity-temperature relationships between the super- and subliquidus regions.
# Egorova N.A.1 , Sushchevskaya2 N.M. , Khvorov D.A.1 , Koptev-Dvornikov1 E.V. and Kononkova2 N.N. Mathematical simulation and specific features of crystallization of tholeiite magmas during the formation of the subaqueous Spiess ridge.
key words [tholeiite magma crystallization]
1(Department of Geology, Moscow State University), 2(V. I. Vernadskii Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences)
The subaqueous Spiess Ridge is located in the South Atlantic Ocean and is the west termination of the South-Western Indian Bridge, which separates the African and Antarctic plates. It was formed under specific conditions when the newly formed rift zone was imposed on the earlier formed ocean crust. Being localized at an angle to the south termination of the Middle-Atlantic Ridge (MAR) and jointing it to the south from the Bouvet island, it had to adapt itself to spreading of the rift MAR zone directed toward. Such a complicated dynamics of the development of the Spiess Ridge region resulted in the fact that a volcanic upheaval with the central type structure formed within it. This upheaval is morphologically similar to a subaqueous volcano with a relative rising about 2 km with minimum marks of depths about 500 m and a caldera about 15 km in diameter surrounded by several secondary small volcanos arranged on the eastern and western slopes of the volcano.
The petrochemical study of the character of magmatism of the Spiess Ridge showed that its formation was accompanied by eruptions of basalts, mainly aphyric, rarely (Cpx)-Ol-Pl-porphyric. The specific feature of basalts is their porosity, which in some varieties reaches 40-60%. The comparison of the petrochemical basalts and glasses from the caldera region, its foot, and at the western flank (performed by the Italian-Russian Scientific "Gelendzhik-96" expedition) revealed that, as a whole, the rocks and glasses of several stations are close in composition and lie on the single trend of the tholeiite differentiation, within the MgO content from 7 to 2.5%. Such an advanced crystallization trend is not mainly typical of magmas formed under low-spreading zones to which the Spiess Ridge is referred and reflects the specificity of its formation. Relatively elevated concentrations of K2O (up to 1.5%) in all lavas of this zone is a distinctive feature of basalts of the Spiess Ridge. For SMAR, a similar tendency is observed near hot points. In addition, a relatively
# This work has been supported by the Russian Foundation for Basic Research (Projects Nos. 96-05-65569 and 96-05-65483).
20
lower content of iron was revealed for glasses of the Spiess Ridge.
A wide range of composition of basalt phenocrysts was established by the microprobe analysis data (Fig. 1), and the most stable associate of the Fo88-An86-Cpx (Mg88) was shown to be equilibrium with more magnesial melts and correspond to the earlier crystallization stage.
The determination of conditions of magma formation under the Ridge, possibilities of existence of an intermediate chamber, and estimation of its parameters are one of the most important conditions for reconstruction of the history of the ocean lithosphere evolution in the region of triple junction of plates. For this purpose, we used numerical simulation of crystallization of basalt melts performed by the KOMAGMAT program (A. A. Ariskin, 1993). The calculation of the crystallization parameters at different pressures (from 0 to 10 kbar) in closed dry system from the least fractionated glass composition to the least magnesial composition showed that none of the trends described satisfactorily the natural variations of the compositions. It has been previously shown (V. A. Simonov et al., 1996) that the glasses from the region of the Bouvet Triple Junction and Spiess Ridge contain elevated concentrations of H2O, which reach 1-1.5% in the most differentiated samples. Therefore, we modeled the crystallization of the melts corresponding to the most magnesial glasses containing about 0.5% H2O. In this case, the fractionation at pressures about 2-3 kbar was the best approximation to the real trend. A water admixture had a strong effect on the liquidus temperatures of the minerals, which resulted in a relative decrease in the iron content in crystallizing melts. The temperature of the melt, which corresponded to the MgO content of 6.5%, was close to 1170oC, and all three minerals were crystallized from this melt as the temperature decreased to 1105oC when titano-magnetite joined them. The results of calculations indicate that an extended intermediate chamber at a depth of 6-8 km exists under the Spiess Ridge. At the same time, primary melts that are generated in the mantle and fall in the chamber are more magnesial than those met on the lava surface. The calculation of the crystallization of the primary melt of the TOR-1 type(tholeiites of ocean rifts) formed during polybaric fractional cumulative melting of the lherzolite ocean mantle within the pressure range of 20-9 kbar and total melting degree of 16-18% (Y. Niu and R. J. Batiza, 1992; N. M. Sushchevskaya and T. I. Tsehonia, 1994) showed that the melts of the Spiess Ridge could be formed during the small-deep crystallization (2-3 kbar) of a similar primary melt with the H2O content about 0.3%. Olivin Fo92 is a liquidus phase during fractionation at 1300oC, plagioclase An86 joins it when the temperature decreases to 1240oC, and an eutectoid association corresponding to the Fo87 An80 Cpx (En48, FS6, Wo45) compositions in the 5:41:54 ratio is crystallized at 1200oC. To this moment, about 35% phenocrysts have fell out of the system. The obtained calculated compositions of the mineral phases coincide with the real composition of the phenocryst minerals.
Fig.1. Histogram of the compositions of phenocrysts of tholeiitic lavas, Spiess Ridge.
Thus, the study of magmatism forming the spreading Spiess Ridge showed that the tholeiite melts erupted on the surface fractionate in a relatively closed system at 3-2 kbar in the 1310-1100oC temperature range from primary melts of the TOP-1 type. Unlike rather dry crystallization conditions typical of the major part of rift zones of the World ocean, the primary melts of the Spiess Ridge contained about 0.3% water and were relatively enriched in incompatible elements.
Fig.2. Position of glasses compositions on the polypressure diagram relative to calculation curves of fractionation for 2.5 and 5 kbar of most magnesial composition at H2O content 0.6 %.
References:
21
# Lebedev E.B. , Kadik A.A. Sorting of crystal upon their precipitation in a magmatic melt: modelling by means of high-temperature centrifuges.
key words [melt crystal sorting centrifuge]
Institute of Geochemistry and analytical chemistry RAS Moscow
Differentiation of magmas is closely related with their crystallization, with the occurrence in the magmatic body of the crystalline phases which are different in density and composition from the melts in equilibrium with them. The separation of these phases in the gravity field of the Earth is the principal mechanism of formation of magmatic rocks of various compositions, the mechanism of this process is however unclear by and large.
The mechanism of differentiation of crystallising magmas being complex, its final result is the accumulation of crystals with the formation of isolated layers (cumulates) within the intrusive. In the process of formation of cumulates a certain amount of the magmatic liquid is trapped, that crystallises upon subsequent cooling under the conditions of closed system or at some diffusional exchange with the basic magmatic body.
Theoretical predictions suggest that the amount of the melt which can be partitioned between cumulate crystals depends on the specific features of the surface energy of the crystals and the silicate liquid. They also determine the crystal-to-melt ratio at which the liquid interrelated channels between the crystals can arise or disappear. This instant of evolution of the melt-crystals mixture is of great interest as it determines the instant in the formation of the cumulative layer when it starts existing as a closed system under the conditions of quite limited mass exchange with the principal magmatic body.
In this work we have attempted to experimentally model the processes of crystals deposition from a silicate melt and the formation by them a cumulative layers using a high-temperature centrifuge. Ingenious centrifuges with the rotation rate 5000-6000 RPM and the rotation radius 120 mm were used. The distance from the rotation center and the number of rotations of the centrifuges made it possible to exceed the Earth's field of gravity by 3000 times. The specific features of the employed high-temperature centrifuges are in the design of a gradient-free rotating furnace and a special collector group with copper-graphite or platinum and mercury contacts. The three-section construction of the furnace ensured a uniform heating of the reaction chamber up to 1400oC. The temperature was measured with three thermocouples. The accuracy of the measurement was +2-3oC at 1200oC .This modelling technique enables one to considerably accelerate the accumulation and sorting of crystals as they are moving in a viscous silicate liquid under gravity. This enables observations of long natural processes at sufficiently short times. The important point of using centrifuges is that this modelling technique does not require a change in the physical properties of liquids and crystalline particles (viscosity, density) in order to retain the similarity with the model subject.
# This work has been supported by the RFBR, grant N 95-05-15292
Table. Melt quantity trapped upon the deposition of crystals and the formation by them of cumulative layers in the runs using as high-temperature centrifuge. The run time is 30 min, the rotational acceleration is 3000g (cm× sec-2). The predominant crystalline phase is given in thick symbols. The starting composition: gbsc-mixture of crystals Pl,Ol,Px,Mt; bsc-basalt.
|
N run |
ToC |
Layer position with respect to the tube height |
Crystalline phases of cumulative layers (in brackets vol% is given) |
Trapped melt vol% |
|
bsc10 |
1200 |
bottom |
Ol(68+3) |
32+3 |
|
bsc12 |
1195 |
bottom |
Ol(69+3) |
31+3 |
|
gbsc21A |
1190 |
top |
Pl(60+2)+Px(1+0.5)+Ol(1+0.5) |
38+2 |
|
gbsc21B |
1190 |
middle |
Mt(33+2)+Px(15+2)+Pl(5+2)+Ol(5+1) |
42+2 |
|
gbsc21C |
1190 |
bottom |
Px(42+5)+Pl(8+2)+Ol(25+5)+Mt(15+2) |
10+2 |
|
bsc28 |
1190 |
bottom |
Px(28+1)+Pl(8+2)+ Ol(2+1)+Mt(10+2) |
52+4 |
|
gbsc18 |
1180 |
bottom |
Px(45+2)+Pl(10+2)+ Ol(20+3)+Mt(15+2) |
10+2 |
|
gbsc17-A |
1180 |
top |
Pl(33+2)+Px(1+0.5) |
66+2 |
|
gbsc17-B |
1180 |
bottom |
Px(45+6)+Pl(8+2)+Ol(4+2)+Mt(12+3) |
31+4 |
|
gbsc20-A |
1180 |
middle |
Px(56+6)+Pl(9+5)+Ol(5+2)+Mt(11+2) |
19+6 |
|
gbsc20-B |
1180 |
bottom |
Px(65+1)+Pl(10+1)+Ol(6+2)+Mt(11+2) |
8+2 |
22
The subjects to study were compositions of mineral mixtures of rocks which make it possible to follow the formation of olivine, pyroxene, plagioclase, and complex olivine-pyroxene-plagioclase cumulates. In our runs the deposition of crystals is performed under isothermal conditions from a melt-suspended crystals mixture in the absence of silicate liquid convective flow. The processes observable in the runs are mainly due to the motion of the phases with respect to each other in the absence (quite limited) of the chemical interaction between them as a result of crystallization or dissolution.
Modelling by means of high-temperature centrifuges shows that the melt quantity trapped by crystals upon their accumulation is 10-15 vol%. (Table). Of the basic magmas crystallization product, viz., olivines, pyroxenes, plagioclases the smallest melt quantity is trapped by olivine crystals at the cumulate formation. This is consistent with the experimental measurements of the specific features of wettability of these crystals with basalt melts. It has been confirmed that pyroxenes are capable of trapping considerable melt quantities. It has been found that at a simultaneous immersion of olivine crystals of different size they form aggregates (cumulative layers) in the melt volume. This is accumulation of another compared with the one forming in the lower parts of the melt.
The modelling using high-temperature centrifuges demonstrates that the polymineral cumulate of olivine, pyroxene, and plagioclase is unstable. Its mineral components the same as the intergranular melt differentiate with the density separation. This process leads to the melt segregation into layers of their own right and to condensation of the crystalline remainder. So, the possibility is confirmed of further differentiation of the deposited crystal layers.
#
Zharkova E.V. , Kadik A.A. , Bibikova E.V. and Troneva M.A. Zircons: Electrochemical determination of oxygen fugacity.key words [zircon oxygen fugacityV. I. Vernadskii Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences, Moscow, Russia]
Experiments on determination of the intrinsic oxygen fugacity of zircons (fO2) from different regions were carried out on a high-temperature setup based on two solid electrolytes of zirconium oxide stabilized by yttrium oxide within the temperature range from 750 to 1100oC and at 1 atm [1]. Description of the samples studied: rapakivi-granites of the Berdyaushskii pluton, Ural (sample 19a). The Berdyaushskii massive is a multiphase pluton, where gabbro, syenito-diorites, rapakivi-granites, nepheline syenites, and heteroaged diabasic dikes are combined on the surface area about 30 km. The accessory zircons studied were taken out from biotite rapakivi-granite. The zircons are exclusively uniform, transparent, light-pink-colored. They are characterized by high idiomorphism, fine zonal structure with the direct geochemical zoning, which indicate magmatic paragenesis. The U-Pb isotopic age of zircons is 1355+10 mln years [2].
Deep seated charnockites of the Baltic Shield (sample 518). The Vichanskii massive of deep charnockites is placed in the joint zone of Belomor and Karel formation of the Baltic Shield. Charnockites are medium-large grained rocks consisting of quartz, plagioclase, Kfps, hypersthene, amphibole, and garnet. The deep genesis of the rocks is evidenced by the presence of xenolites of pyroxenites and gabbroids, the blue color of quartz, and excessive concentrations of inert gases in rock-forming minerals. For example, the K-Ar age of biotites is up to 4.0 bln. years. The form of zircon grains also indicates their crystallization under deep conditions. They are fine, transparent, lustrous, and strongly flattened grains. The content of uranium in zircons is very low. The U-Pb age of zircons is 2400 + 20 mln. years [3].
Gabbro, Orekhovo-Pavlograd Zone of the Ukrainian Shield (sample 29950). The Orekhovo-Pavlograd submeridional stripped structure separates the Pridnestrovskii and Priazovskii blocks of Precambrian rocks. It is restricted by the western Orekhovo-Pavlograd and eastern Azovo-Pavlograd deep fractures, which, according to the geophysical data, cross the Earth crust and go into the upper mantle. Intrusive rocks of the ultrabasic and basic compositions are widely developed within the structure. During many years, they were considered to be in the composition of the lower Proterozoic era. Accessory zircons are presented by isometric colorless, transparent, and exclusively low-uranium grains without crystallographic forms. These zircons are usually crystallized in the basic composition rocks at the last stages of the magma crystallization. The accessory zircons studied were recovered from gabbro with the age of 2650 + 20 mln. years.
Granitoids of Pridnestrov'e (sample 650). The region of the Middle Pridnestrov'e is a classic region of developed rocks of the granite-green stone associate. The accessory zircons studied were isolated from quartz diorite of the Yamburg open pit on the left shore of the Mokraya Sura river. The zircons are predominantly from long-prismatic to needle-like forms, pink-brown colored. Concretions of two and more grains along the long axis are observed. The fine intrinsic zonality indicates the magmatic genesis of zircons, and no internal nuclei were observed. The accessory zircons have the low content of uranium and the high value of the Th-U ratio. The U-Pb age of the zircons is 3000 + 20 mln. years [3].
Orthogneisses of the Omolonskii massive (sample Chuk 10). Orthogneisses of the Omolonskii massive (North-East Siberia) are one of the most ancient in Russia. They form the Aulandzhinskii ridge 7x15 km in size. These gneisses are highly metamorphosed, mainly with the tholeiite composition. The accessory zircons in the tholeiite gneiss studied are mainly presented by heteroelongated subidiomorphically prismatic brown grains. The crystals are semitransparent, some of them retain traces of fine zoning, which indicates the primary magmatic origin. The data obtained for the accessory zircons
# This work was financially supported by the Russian Foundation for Basic Research (Project No. 96-05-64954).
23
indicate that the ages of the rocks is not less than 3.5 bln. years [3].
The experiments performed (see table) showed that fO2 of the zircon crystals lies in the area of the buffer equilibrium WM (wüstite-magnetite). However, for "younger" zircons (samples 19a, 518, and 29950) at temperatures 1100oC and higher, the line corresponding to the intrinsic oxygen fugacity of the samples listed crosses the buffer QMF line (quartz-magnetite-fajalite). The intrinsic oxygen fugacity obtained for more "ancient" zircons (samples 650 and Chuk 10) is arranged along the line of the buffer equilibrium WM or slightly lower ~ 0.5 fO2 units).
We attempted to consider the dependence of the intrinsic oxygen fugacity of the zircons studied on their age, but no pronounced correlation was observed.
Table 1. Coefficients "A" and "B" in the empirical dependence logfO2 = A-B/T for zircons from different regions
|
Sample |
A |
B |
n |
r |
|
19a |
20.906 |
42224.5 |
9 |
0.998 |
|
518 |
18.808 |
39030 |
13 |
0.983 |
|
650 |
12.351 |
32044.3 |
10 |
0.983 |
|
29950 |
27.576 |
50453.2 |
9 |
0.987 |
|
Chuk 10 |
14.463 |
35616.8 |
9 |
0.994 |
Note: r is the correlation coefficient; n is the number of experimental points.
References:
Kotelnikova1 A.A., Rusakov1 V.S., Bychkov A.M.2 Mössbauer study of the influence of the melting temperature on the structural and valence state of iron in natural and synthetic silicate glasses.
key words [iron valent state melt ]
Mossbauer spectroscopy methods have been used to investigate natural and synthetic silicate glasses. The aim of this work was to determine the structural and valence states of iron in natural and synthetic silicate glasses produced at various temperatures of the initial melt. Natural glasses - tengizites (tz glasses ) formed during the petroleum fire (the combustion period was about a year) in one of the boreholes of the Tengiz deposit (Kazakhstan). The temperatures of the melt from which the glasses formed range 2600 to 3000oC. The chemical composition of the glasses complies with that of andesite with a strongly increased concentration of CaO and a lower concentration of FeO. The synthetic glasses are compositionally close to the mineral kirsteinite CaFeSiO4 (kr glasses obtained from melts having the temperatures 1200-1400oC) and to ferrisilicate feldspar KFeSi3O8 (fs glasses obtained from melts having the temperatures 1050-1350oC). The melts were kept at the appropriate temperature for no longer than 2 h. The processing and analysis of the Mossbauer spectra using the program DISTRI, developed by us, were performed through resetting the independent functions of the distribution parameters of the hyperfine interaction of 57Fe nuclei, corresponding to Fe2+ and Fe3+ ions. The obtained distribution functions were analyzed by means of the one-modal distribution characteristics. The value state and the dominant coordination of Fe ions were determined from the mean isomeric shift value, the occurrence of other coordination states was determined from its distribution width. The mean value of the quadrupole shift and its distribution width made it possible to estimate the degree of distortion of the oxygen polyhedrons of Fe atoms and the variations of their junction angles.
The studies performed suggest the following conclusions. In natural tz glasses Fe2+ and Fe3+ ions are present in approximately the same amounts. Fe3+ occupies octahedral positions. Fe2+ ions occur only in the state of coordination number 5. This fact is associated with the specific features of the chemical composition of tengizites, namely, with the high content of the cation-modifier Ca2+ (CaO~ 17¸ 18 wt%) and a rather high content of the cation-net former Al3+ (Al2O3 ~ 9 wt%). As the temperature of the melt is increased from 2600 to 3000oC, the share of Fe2+ increases by several percents but the structural state remains unvariable. Kr glasses comprise bivalent and trivalent iron ions in the ratio Fe2+:Fe3+ » 1:6.
The coordination number of Fe3+ ions is 6. The oxygen octahedrons are strongly distorted, the degree of distortion changes from site to site. The mean value of the isomeric shift for Fe2+ ions is between the values characteristic of tetrahedral and pentahedral coordinations. The greater distribution width o the hyperfine parameters bears witness to the fact that Fe2+ ions occupy the positions of both these types. No changes are observed in the coordination state of iron with the growing temperature. In fs glasses iron is present only in the trivalent state in the tetrahedral coordination. However, the broad distribution of the hyperfine parameters indicates that even for the oxygen tetrahedrons of the closest neighborhood of Fe3+ ions strong deformations and variations of their junction angles are characteristic. The iron does not alter its valence state with the growing temperature of the melt, but during this event the degree of the distance distortion in the oxygen polyhedrons grows.
So, with virtually invariable Fe2+ :Fe3+ ratio the coordination states of Fe ions are independent of the tem
24
perature of the silicate melt. However, an increase of the degree of the distance distortion in the oxygen polyhedrons can be observed, the mean lengths of the Fe-O bonds being invariable. Under the conjecture that during the melt  glass transition only small changes occur in the closest neighborhood of Fe ions, the obtained results can be extended for silicate melts as well.
glass transition only small changes occur in the closest neighborhood of Fe ions, the obtained results can be extended for silicate melts as well.
#
Durasova N.A. , Kochnova L.N. , and Belyaeva V.K. Migration properties of copper in synthetic analogs of volcanogenic rocks under reduction conditions at sub-solidus temperatures.key words [copper migration rock]
V. I. Vernadskii Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences, Moscow, Russia
Our previous studies of the behavior of copper in alumosilicate systems as the oxygen potential increased showed that the state and migration properties of the metal depended on this value [1, 2]. The specificity of the behavior of the ore element during the growth of the hydrogen pressure (hydrogenolysis), which is also real for natural systems, remained unstudied.
Based on the results of studying by ESR, ESCA (electron spectroscopy for chemical analysis), X-ray spectral microanalysis, and atomic absorption of alumosilicate glasses with compositions of the main types of volcanogenic rocks synthesized at liquidus temperatures and fO2 of the Ni-NiO buffer and in air, Table 1 (according to the procedure described in [1]) on heating under reduction conditions, we established that
- copper is present in the alumosilicate phases predominantly in low oxidation states (Fig. 1);
- its redistribution accompanied by the formation of copper-enriched layers on the grain surface (Table 2, Figs. 2 and 3) is observed due to the instability of the Cu2+/Cu+ ratios in the starting glasses on heating at 500oC;
- considerable (by more than an order of magnitude) copper loss by hydrochloric solutions is observed upon the interaction with the phases underwent heating at 500oC and the Ni-NiO buffer as compared to the loss from the starting samples (Table 1).
These facts can indicate that the ore-generating ability of volcanogenic rocks under natural conditions similar to the experimental parameters in the case of changing redox conditions (both decreasing and increasing the oxygen potential) can increase considerably.
Fig.1. Copper EPR spectra in the glasses 1-234 (in the air); 2-234-500 (in the air); 3 - 234-500(the buffer Ni- NiO)
Fig.2. ESCA spectra in the sample 232-2-500 (in the air).
# This work was financially supported by the Russian Foundation for Basic Research (Project No. 95-05-150-68a).
25
Table 1. Data on the copper loss from glasses by solutions of HCl (5N) contacted with them
|
Sample no. |
Type of glasses |
Conditions of synthesis, heating, and washing of glasses |
Copper content in a solution of HCl after washing of glasses/ml; |
Copper content in glass* |
Copper loss from glasses |
||||
|
ToC |
fO2 |
Duration of synthesis and heating; |
Weighed sample of glass. mg |
Volume of solution, ml**; |
|||||
|
215 start*** |
granite eutectic |
1500 |
air |
0.5 h |
47.4 |
2 |
2.6 |
0.34 |
3.0 |
|
234 start**** |
granite eutectic |
1150 |
air |
3 h |
47.9 |
2 |
1.1 |
0.34 |
1.5 |
|
233 1start***** |
Deli granite |
1000 |
Ni-NiO |
12 h |
56.1 |
2 |
2.4 |
0.33 |
2.7 |
|
232 1start***** |
hawaiite |
1200 |
Ni-NiO |
6 h |
48.6 |
2 |
0.2 |
0.40 |
0.3 |
|
232 2start***** |
hawaiite |
1100 |
Ni-NiO |
24 days |
47.0 |
2 |
0.4 |
0.40 |
0.3 |
Note:
*Data of atomic absorption (analyzed by T. V. Shumskaya).
** Contact time of samples and solution was 40 min.
*** Glasses were synthesized in a lanthanum furnace at T = 1500oC (before heating of the glasses, ESR showed no Cu2+ in the glasses because of decomposition
2CuO  2CuO+1 + O2 at T > 1500oC).
2CuO+1 + O2 at T > 1500oC).
**** Glasses were synthesized in a flame furnace (ESR showed that Cu2+ was in the glasses before heating).
***** Glasses were synthesized in a muffle furnace. The fO2 values for the Ni-NiO buffer mixture (L. N. Kurshakova, 1976): -21.68(527oC); -9.903(1027oC); -8.56(1127oC); -6.82(1227oC).
26
Table 2. Atomic concentrations of elements in the surface layer of glasses (%) (by the ESCA data on a PH I 5400 instrument, analyzed by A. V. Shchukarev)
|
Sample no |
C1s |
Ca 2p |
Al 2p |
Na 1s |
Mg 1s |
Al2 s |
|||||||
|
O 1s |
Si 2p |
Fe 2p |
Ti 2p |
Cu 2p |
K 2p |
||||||||
|
215 start |
41.84 |
32.43 |
- |
20.00 |
2.97 |
- |
2.05 |
- |
- |
- |
- |
0.72 |
|
|
215-500 (air) |
24.22 |
38.90 |
- |
24.63 |
6.24 |
- |
4.85 |
- |
- |
- |
- |
1.16 |
|
|
215-500(Ni-NiO) |
84.13 |
9.02 |
- |
5.41 |
0.79 |
- |
0.55 |
- |
- |
0.09 |
- |
- |
|
|
234 start |
33.59 |
39.15 |
- |
21.82 |
2.87 |
- |
1.75 |
- |
- |
- |
- |
0.82 |
|
|
234-500 (air) |
48.01 |
28.68 |
- |
16.16 |
3.57 |
- |
2.44 |
- |
- |
0.59 |
- |
0.55 |
|
|
234-500 (Ni-NiO) |
34.70 |
37.44 |
- |
20.22 |
- |
- |
2.40 |
- |
- |
4.45 |
- |
0.79 |
|
|
233-1 start |
67.57 |
18.83 |
0.28 |
9.92 |
2.80 |
0.17 |
0.44 |
- |
- |
- |
- |
- |
|
|
233-1-500 (air) |
43.56 |
32.19 |
- |
17.81 |
- |
0.41 |
0.91 |
- |
- |
1.28 |
3.12 |
- |
|
|
233-1-500 (Ni-NiO) |
80.77 |
10.68 |
- |
6.30 |
1.65 |
- |
0.37 |
- |
- |
0.23 |
- |
- |
|
|
232-1 start |
12.41 |
50.96 |
2.37 |
20.02 |
9.58 |
2.67 |
1.03 |
0.95 |
- |
- |
- |
- |
|
|
232-1-500 (air) |
26.88 |
37.67 |
- |
13.64 |
- |
2.67 |
4.90 |
0.63 |
- |
6.10 |
7.51 |
- |
|
|
232-1-500 (Ni-NiO) |
58.61 |
22.67 |
0.76 |
9.19 |
- |
0.59 |
1.37 |
- |
- |
3.19 |
3.61 |
- |
|
|
232-2 start |
63.63 |
20.51 |
1.21 |
7.98 |
4.52 |
1.16 |
0.65 |
0.31 |
- |
- |
- |
- |
|
|
232-2-500 (air) |
51.00 |
18.93 |
- |
- |
- |
- |
- |
- |
- |
30.07 |
- |
- |
|
|
232-2-(Ni-NiO) |
85.52 |
8.30 |
0.41 |
3.89 |
1.48 |
0.16 |
0.24 |
- |
- |
- |
- |
- |
|
References:
27