V. Hydrothermal fluid systems
Zharikov V.A. Fluids in geological processes.
Detailed experimental and theoretical study of phase relations in the model K2O-Al2O3-SiO2-H2O system as well as mineral and phase relationships in some magmatic and metamorphic complexes showed the principle distinctions of dehydration and melting reactions under different water regimes. The analysis of natural material in this context showed the failure of any conceptions implying inert behaviour of water in endogenic processes, including the melting-dehydration conception, which is so popular among non-Russian scientists (Zharikov, 1995).
Specific properties of fluids in fine-porous natural media are paramount for understanding hydrothermal processes. At such conditions, component activities differ markedly from those in bulk solutions because of surface effect. In particular, mole fraction of H2O in metamorphic processes is significantly higher, than that calculated from metamorphic reactions because [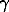 pore (CO2)>1> pore (H2O)] (Zharikov V.A., Shmonov V.M., Shmulovich K.I.). The filtration effect, caused by electrokinetic phenomena or possible component partitioning on heterostatic fluid transport, has affect significantly the transfer of components (Zharikov V.A., Koshemchuk S.K.).
pore (CO2)>1> pore (H2O)] (Zharikov V.A., Shmonov V.M., Shmulovich K.I.). The filtration effect, caused by electrokinetic phenomena or possible component partitioning on heterostatic fluid transport, has affect significantly the transfer of components (Zharikov V.A., Koshemchuk S.K.).
References:
- Zharikov V.A. Fluids in Geological processes. // Fluid in the Crust. Eds. K.I.Shmulovich, B.W.D. Yardley, G.G.Gonchar. London. Chapman & Hall, 1995. pp.13-42.
- Zharikov V.A. Dehydration and melting reactions at the different water regimes. // Petrology, 1995. V.3, No.4, pp.340-348 (in Russian).
Alekhin Yu.B., Plyasunov A.V. Development of theories of fluid equilibria.
A method was developed that makes it possible to correlate the degrees of homogeneous molecular hydrolysis reactions in low-density vapor and the thermodynamic functions and concentrations of halide hydrates and hydroxide hydrates, which form as a result of hydrolysis. Experimental studies of Ca(OH)2 and Fe2O3 solubilities in water vapor were initiated to verify the assumptions, on which the method is based.
A method for the determination of Henry constants for monophase supercritical fluids (to 400-800oC, 5 kbar) was developed by considering Henry constants as standard fugacities of dissolved molecular species in dilute solutions. Thermodynamic properties of fluid components of gas- water systems were calculated. The results were compared with experimental data on the H2O-CO2 system.
Standard thermodynamic properties of NaCl at hydrothermal parameters were evaluated in terms of the total equilibrium constantþ conception on the basis of the six-parameter model for determination of partial molar properties of dissolved electrolytes. The standard properties of NaCl, i.e. molar volumes, compressibilities, isobaric heat capacities, entropies, enthalpies, and Gibbs energies, were calculated for pressures to 4 kbar and temperatures 0-400oC with accuracy close to that achieved in experiment (at least 0.05% for volumes). There are reasons to suppose that the proposed method of calculation of solution properties is valid up to 1000oC and 10 kbar.
35
Plyasunov A.V. Development of thermodynamic complexation models based on the concentration dependences of the coefficients of interspecies interaction.
The temperature dependence of the stability constants of all the complex compounds (mononuclear and polynuclear) forming in a M-L system has been described. In the procedure, only one fit parameter (M-L bond length) was used, which was assumed to be the same for all the species. The formation of polynuclear compounds consists of two processes: formation of mononuclear compounds and simple ions (energies were evaluated according to Ryzhenko and Bryzgalin model) and formation of the polynuclear aggregate from the mononuclear constituents (energy was expressed as the sum of electrostatic and nonelectrostatic components).
The methods of description of concentration dependences of complexation equilibrium constants were overviewed. It was shown that the Pitzer method, which is commonly applied for the interpretation of experimental data in terms of activity coefficients, partial and apparent molar characteristics of ordinary elecrolytes and their mixtures, is badly suited for the description and calculation of log Ko at infinite dilution because of a great number of regression parameters (insufficiently specified task in terms of the least-squares method). A system of equations consistent with the postulates of specific interaction theory (SIT) was derived for the description of the concentration dependences of osmotic coefficients of binary elecrolytes, electroneutral combination of an ion and neutral species mixure, and partial and apparent properties of electrolytes[5].
Lakshtanov L.Z. The model of two-phase filtration in the H2O-CO2 system.
1. The model was developed for the matter separation in two-phase filtration of solutions through a fine-porous medium [1, 2]. It was shown that the filtration effect can be many times increased in filtration of heterogeneous fluids. A simple case of isothermic steady-state heterophase filtration was considered when there is no chemical interaction between the porous hydrothermal solution and wall rock. The proposed model involves the system of differential Darcy equations for the densities of mass fluxes of components. The model takes into account the limiting pressure and initial compositions of the solution and is based on functional thermodynamic and physicochemical relationships determining local heterogeneous equilibrium in the system (relations of pressure and phase compositions on the surface of the heterophase immiscibility dome; dependences of fluid density and viscosity on composition and pressure; dependence of relative phase permeability on phase saturation was assumed to be linear).
The computer modeling revealed a significant increase of carbon dioxide concentration in the fluid at the end of the filter, even in case of significantly aqueous compositions of the initial fluid. The phase saturation changes dramatically at the begining of the heterogenization. Water saturation markedly decreases at the end of the filter in the filtration of significantly aqueous fluid; the more pronounced pressure gradient, the more significant water-saturation decrease. In case of filtration of significantly carbonate fluids, water saturation slightly increases at the end of the filter, but remains lower than 0.1 under any conditions.
2. The effect of two-phase filtration on the separation of solution components via the filtration effect mechanism (electrokinetic and barodiffusion) was studied on the basis of the model described above. The computer modeling showed that the distribution of electrolyte in porous medium in two-phase filtration markedly differs from that in single-phase filtration and has an extremum (the maximum electrolyte concentration corresponds to the flow front). The two-phase filtration effect significantly excels the single-phase filtration effect.
In two-phase filtration, the more pronounced separation via barodiffusion mechanism results from the significant decrease of the permeability constant of the liquid phase with proportional decreasing water saturation. The concentration distribution is extremal, as in electrokinetic process, with the maximum attained at the front of the filter. 3. The filtration model for the pseudobinary system water-carbon dioxide was proposed. The model takes into account hydrolysis of NaCl, which is present in the fluid in insignificant amount (less than 0.01 wt %). The computer modeling results indicate that within wide ranges of pressure (200-500 bar; T= 300oC) and starting fluid compositions, HCl concentration decreases at the end of the filter, but remains higher than NaOH concentration at any section of the porous filtration column.
References:
- Koshemchuk S.K., Alekhin Yu.V., and Lakshtanov L.Z. (1994) The results of heterophase filtration studies. // Experimental problems of Geology., Moscow: Nauka, pp.556-570.
- Koshemchuk S.K., Magomedov M.A., Alekhin Yu.V., and Lakshtanov L.Z. (1995) Separation processes in the stationary two-phase filtration in gas-water systems. // Geokhimiya, N.3, pp.441-455.
Lakshtanov L.Z. Experimental study of disproportionation processes in hydrothermal recrystallization.
1. Coprecipitation and adsorption of ore components from unsaturated ore- bearing solutions may be predominant in the formation of quartz-gold, quartz- cassiterite, and gold-sulfide deposits. To evaluate the degree of concentration of impurity components during the mineral crystallization from gel-phase, theoretical modeling of coprecipitation and further recrystallization of the formed mineral under hydrothermal conditions was carried out. The model is based on the principle of irreversible isolation of already formed mineral phase from the hydrothermal fluid. Such conditions correspond to the rapid crystallization of a mineral from the gel-phase or recrysrallization of a dispersed phase on aging. The theoretical modeling yielded the following results. The dependence of the de-
36
gree of impurity concentration on the mass of precipiated or recrystallized dispersed mineral phase was determined. The dynamics of redistribution of a primarily adsorbed impurity during the recrystallization of matrix mineral was studied. The theoretical model applied to experimental data on the distribution of oxygen isotopes in quartz synthesis from gel-phase in the presence of aqueous and brine (NaCl) fluids made it possible to determine the coefficients of local distribution of an isotope-substituted component between the boundary layer of a growing crystal and fluid. It was established that the coefficient of local distribution depends on crystal size. Excessive impurity adsorption, compared to equilibrium adsorption, was established at 400-600oC and 1000 bar, in the crystal radius range 100-200 mm,. This effect is significant when a mineral crystallizes from gel-phase. The behavior of isotope-substituted 18O-bearing silica molecule was experimentally studied [1]. The described mechanism may work in concentration of dispersed impurity elements during the recrystallization of gel-phases of matrix minerals (quartz) in hydrothermal ore systems (E.O. Dubinina and L.Z. Lakshtanov).
2. An experimental series of potentiometric titrations of SiO2-gel suspension in NaCl solutions was performed to study acid-base properties of amorphous silica surface. 250 experimental points were obtained in 9 runs. The dependence of the surface charge density on pH was experimentally studied:
log[-SiO-]=3,02log(pH) - 7,10.
The obtained results: log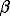 -1,1(int) = -3.5, log
-1,1(int) = -3.5, log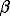 -1,1(int)=7,5 and zero point charge (ZPC) pH=2 are in agreement with other published data.
-1,1(int)=7,5 and zero point charge (ZPC) pH=2 are in agreement with other published data.
3. Titrations of silica suspension in HAuCl4 solution (background electrolyte NaCl) were conducted to study adsorption of gold on amorphous silica. The gold concentration was determined by colorimetry and AAS in solution aliquots sampled from the potentiometric cell at every point of acid-base titration. Adsorption of gold in the AuCl4 form was shown to increase drastically with increasing pH from 1.7 to 2. On further pH increase, two adsorption maxima (pH 4.3 and 7.5) are fixed, which correspond to the constants of hydroxyl-chloride complex compound formation:
AuCl-4 + H2O = AuCl3OH- + H+ + Cl-
AuCl3OH- + H2O = AuCl3 (OH)2- + H+ + Cl-
Reference:
- Dubinina E.O., and Lakshtanov L.Z. (1994) An investigation of oxygen isotope exchange at the synthesis of the mineral under the hydrothermal conditions; theoretical modeling and experiment in the quartz-fluid system. // Experiment in GeoSciences, V.3, N.4, pp.1-19.
Alekhin Yu.B., Vakulenko A.G. The study of solubility and solvation in solid-gas equilibria.
The Fe2O3 solubility in water and dilute HCl solutions was studied at 400-500oC and 300-500 bar. The experiments showed that at 400o C the solubility slightly increases in the pressure range 300-500 bar. The solubility increases almost by a factor of 100 with increasing HCl concentration from 10 to 100 mol/kg, which is consistent with other published data. The hydration number of Fe2O3 in neutral solutions is supposedly closed to unity.
The Ca(OH)2 solubility in water vapor was studied at 350-500oC and 30-300 bar. An invrese dependence of the solubility on pressure, as predicted by the Gibbs-Dalton equation for gaseous solutions of nonvolatile compounds at low pressures, was first experimentally obtained for water vapor solutions. This fact testifies to the insignificant hydration of Ca(OH)2 in water vapor, which is contrary to the existence of quadrihydrates in dense solutions at the same temperatures established by Wolter. The comparison of these results with Wolter's data on dense phase testifies that the Ca(OH)2-H2O system is a II-type system with critical phenomena in saturated solutions. The parameters of the SLG-equilibrium are close to those of the LG-equilibrium of water.
The experimental study (T = 200-600oC and P = 0.5 -1.0 kbar) of the dissolution kinetics and solubility of quartz in water was completed. The surface energy contribution was first determined at high PT-parameters. It was shown to increase regularly with decreasing temperature and increasing pressure, which can be explained by changing Gibbs' water adsorption on hydrophile mineral surface [3, 4] (L.Z. Lakshtanov, V.M. Shmonov, and T.P. Dadze).
References:
- Koshemchuk S.K., Alekhin Yu.V., and Lakshtanov L.Z. The results of heterophase filtration studies // in Experimental problems of Geology, Moscow: Nauka, 1994, pp. 556-570.
- Lakshtanov L.Z. Thermodiffusion in electrolyte solutions under hydrothermal conditions // in Experimental problems of Geology, Moscow: Nauka, 1994, pp. 570-578.
- Lakshtanov L.Z., Shmonov V.M., Dadze Y.P., Van der Erden A., and Yansen B. Surface free energy of the quartz-water interface at hydrothermal conditions // Dokl. Ross. Akad. Nauk, 1994, vol. 338, nos. 1-2, pp. 89-91.
- Lakshtanov L.Z., Shmonov V.M., Dadze T.P., Van der Erden A., and Yansen B., Surface free energy of the quartz system at high temperatures and pressures // Geokhimiya, 1994, no. 10, pp. 1479-1488.
- Plyasunov A.V. and Grenthe I. The temperature dependence of stability constants for the formation of polynuclear cationic complexes // Geochim. Cosmochim. Acta, 1994, vol. 58, no. 17, pp. 3561-3582.
- Koshemchuk S.K., Magomedov M.A., Alekhin Yu.V. and Lakshtanov L.Z. Separation processes in the stationary two-phase filtration in gas-water systems // Geokhimiya, 1995, no. 3, pp. 441-455.
- Dubinina E.O. and Lakshtanov L.Z. An investigation of oxygen isotope exchange at the synthesis of the mineral under the hydrothermal conditions; theoretical model
37
ing and experiment in the quartz-fluid system // Experiment in Geosciences, 1994, vol. 3(4), pp. 1-19.
Gorbaty Yu.E., Kalinichev A.G., Bondarenko G.V. Experimental and theoretical studies of the physical and chemical properties of supercritical aqueous fluids.
The work has been completed on the establishment of experimental foundation for the fluctuation theory of liquid state. It is determined that the "structure" of liquids is defined by the wide spectrum of all possible fluctuations (molecular configurations) of the nearest neighbors. These structural fluctuations, in turn, are resulting from the fluctuations of the thermodynamic parameters. Depending on the external conditions, energetically more favorable fluctuations can exist for a longer time, or occur more frequently, i.e., the probability of their existance is higher. It was possible to quantitatively demonstrate that under the thermodynamic conditions close to phase boundaries the most favorable structural fluctuations are those corresponding to the structure of the neighboring phase [5].
The properties of water - the most important component of the hydrothermal fluid - are determined by the ability of water molecules to form hydrogen bonds with each other. These bonds make significant contributions to the thermodynamic properties of water and aqueous solutions. Therefore, for the first time ever, the attempt has been made to determine the enthalpy of H-bond formation directly at high temperatures (up to 450oC) using the technique of infrared absorption spectroscopy. The attempt turned out to be very successful, and the results obtained are already used in a wide range of problems [1]. Computer modeling was used to study the configuration contribution to the thermodynamic properties of isotope-substituted water dimers (HDO)2 [2].
A new phenomenological approach has been developed to the description and prediction of the non-ideality effects for supercritical mixtures of non-electrolytes [ 3].
The properties of supercritical water within the range 573<T<1273 K and 0.1<P<100 kbar were studied by Monte Carlo (MC) and Molecular Dynamics (MD) methods of computer simulation. Simulated values of enthalpy and internal energy, density, heat capacity, isothermal compressibility, thermal expansion and self-diffusion coefficients agree well with available experimental data. The simulated water structure and vibrational spectra are also in good agreement with experimental data, including those obtained earlier at IEM. The success of the present simulations encourages us to expect these methods to be able to predict the properties of more complex aqueous fluids on the fundamental molecular level under the thermodynamic conditions covering the entire range of temperatures, densities, and compositions characteristic of hydrothermal and metamorphic processes [4, 8].
Based on the detailed analysis of all available spectroscopic, diffraction, and thermodynamic experimental data obtained over the last 20 years in several laboratories (including IEM RAS), it was conclusively demonstrated that hydrogen bonds can exist in supercritical water at least up to temperatures of 800 K in the wide range of densities from dilute vapor to compressed liquid[7]. Based on simple geometric, energetic and temporal criteria a new reliable method has been developed to quantitatively analyze the parameters of hydrogen bonding in water at high temperatures and pressures. Compared to H-bonds in ambient liquid water, the H-bonds at 773 K are, on average, 10% weaker, 5% longer, and more bent. The average lifetime of an individual H-bond under supercritical conditions is about an order of magniture lower than the same parameter for liquid water at room temperature and pressure [8].
Pioneering experiments have been completed on the in situ Raman spectroscopic studies of the system S-H2O at a pressure of 1 kbar and temperatures up to 500oC. Preliminary estimates of the concentarions of various form of sulfur in hydrothermal solutions show that the prevailing forms are H2S, SO2, [HSO4]- and elemental sulfur. Temperature dependencies of concentrations for all major components in the solution have characteristic extrema around the critical isotherm which corresponds to the imaginary bordering line separating the region of liquid-like state of the supercritical aqueous phase from the region transitionary to the gas-like state [6].
References:
- Bondarenko G.V., Gorbaty Yu.Eu. (1991) An infrared study of water vapour in the temperature range 573-723 K. Dimerisation enthalpy and absorption intensities for monomer and dimer. // Mol. Phys., V.74, pp.639-647.
- Slanina Z., Kalinichev A.G. (1991) A configurational contribution to the heat capacity of gaseous (HDO)2. // Z.Naturforsch, V.46a, pp. 39-42.
- Kalinichev A.G. (1991) Large excess volumes of supercritical fluid mixtures: A thermodynamic explanation. // Plinius (Suppl. Ital. Eur. J. Mineral.), N5, pp.118-119.
- Kalinichev A.G., Heinzinger K. (1992) Computer Simulations of Aqueous fluids at high temperatures and pressures. // In: Thermodynamic Data. Systematics and Estimation. Ed. Saxena S.K., Adv. Physical Geochemistry, V. 10, Springer Verlag Pub., pp.1-59.
- Okhulkov A.V., Demianets Yu.N., Gorbaty Yu.E. (1994) X-ray Scattering in Liquid Water at Pressures up to 7.7 kbar: Test of a Fluctuation Model. // J.Chem.Phys., V.100, pp.1578-1587.
- Gorbaty Yu.E., Bondarenko G.V. (1995) High-pressure high-temperature Raman cell for corrosive liquids. // Rev. Sci. Instruments, V.66, pp.4347-4349.
- Gorbaty Yu.E., Kalinichev A.G. (1995) Hydrogen bonding in supercritical water. I. Experimental results. // J. Phys. Chem., V.99, pp. 5336-5340.
- Kalinichev A.G., Heinzinger K. (1995) Molecular dynamics of supercritical water: A computer simulation of vibrational spectra with the flexible BJH potential. // Geochimica et Cosmochimica Acta, V.59, pp. 641-650.
38
Shmulovich K.I., Plyasunova N.A., and Tkachenko S.I. Phase relations in fluid-salt systems.
The PT-x conditions of fluid heterogenization in the systems H2O-CO2-salt (NaCl, KCl, CaCl2, MgCl2) were determined at 400-600oC and 3-5 kbar. The experimental studies showed that the natural metamorphic fluid consists of two immiscible phases: water-salt and CO2-methane-nitrogen. The mean salt concentrations (10-20 wt %) are sufficient to provide the heterophase fluid in metamorphism and migmatization. The interaction of the magmatic fluid with carbonates in skarn formation results in the expansion of its heterophase field, decreasing the critical temperatures of isobaric homogenization, and formation of "acidic" (Phase G) and "alkaline"(Phase L) solutions.
The parameters of the liquid-vapor equilibrium and phase compositions in the binary systems water-salt (NaCl, KCl, CaCl2, MgCl2) were measured over the temperature range 400-600oC and pressures to 1.6 kbar. The critical parameters for the solutions in these systems were also determined [1, 2]. The substitution of Na for K does not change the immiscibility parameters of the systems; the substitution of monovalent for bivalent cations reduces the critical temperature, increases the critical pressure, and expands the heterophase field. The investigation of the ternary systems H2O-CO2-salt (CaCl2, NaCl) at 3-5 kbar and 500 and 700oC was completed. The temperature, pressure, and concentration dependences of the heterophase field boundaries of the model H2O-CO2-salt fluid were studied, and the effect of salt component (NaCl or CaCl2) on the immiscibility parameters was examined. The Henry relationship (the proportionality of gas solubility in a liquid to the gas pressure) proved to be inverted at pressures about 3 kbar. CO2 solubility in the water-salt fluid increases with pressure at P < 3 kbar, but decreases at P > 3 kbar. This phenomenon is not only typical of concentrated salt solution-melts, but also the solutions undersaturated at room temperatures. The pressure dependences of CO2 solubility in CaCl2 and NaCl solutions are similar, however, the salt action of CaCl2 is naturally stronger. The expansion of the heterophase field at high pressures have the following consequences:
1. The CO2-phase coexists with the water-salt phase at amphibolite and granulite facies conditions, with the CO2-rich phase appearing as pure CO2 in fluid inclusions.
2. The fluid keeps its heterophase constitution in migmatization and anatexis melt crystallization at any parameters of crustal processes.
3. The CaCl2 or MgCl2 concentrations in the liquid phase increase (i.e. Na and K are substituted for Ca and Mg in the solutions, for instance, in skarn formation). This leads to the expansion of the liquation field, breakdown of the fluid into two phases, and decreasing CO2 concentrations in the water phase.
The water activity in water-salt systems was found to decrease drastically with increasing pressure. This effect was experimentally calibrated in the system H2O-NaCl at pressures up to 10 kbar and 80 mol % of water in the fluid (H2O activity less than 0.3). The decreasing water activity with increasing pressure at isochemical conditions explains the existence of "dry" mineral parageneses in highly metamorphosed rocks (granulites, charnockites, eclogites). The study of albite melting at NaCl-solution pressure of 9 kbar was completed. The position of the melting curve of low albite at 800oC and pressure of 9 kbar created by the H2O-CO2 mixture was verified to standardize the salt effect of albite melting. The obtained data on albite melting at 9 kbar allowed us to calculate the activity coefficients of water in NaCl solutions up to 9 kbar and 1000oC and refine the conditions of anatexis melting.
References:
- Tkachenko S.I. and Shmulovich K.I. (1992) Dokl. Akad. Nauk SSSR, no. 6, vol. 326, pp. 1055-1059.
- Shmulovich K.I., Tkachenko S.I., and Plyasunova N.V., (1995) // Fluid in the Crust: Shmulovich, Yardley, and Gonchar, Eds., pp. 193-214.
- Shmulovich K.I., and Plyasunova N.V. (1993) Geokhimiya, no. 5, pp. 666-684.
- Plyasunova N.V., and Shmulovich K.I. (1991) Dokl. Akad. Nauk SSSR, vol. 319, pp. 378-741.
Korzhinskii M.A. The behaviour of nondissociated HCl in the dense fluid with buffered HCl activity.
The study of HCl behavior in equilibrium with the buffer assemblage Ag-AgCl-Fe3O4-Fe2O3 in water-gas-salt mixtures at T<400oC was completed. There is an inverse correlation between the molecular HCl fugacity and its volume concentration at water density more than 0.85 g/cm3. HCl concentration decreases with increasing pressure and HCl fugacity. This phenomenon is similar to the expansion of the immiscibility field with increasing pressure in the system water-nonpolar gas (Smulovich et al.) and is evidently due to the structural transition of water while its density increases.
The salt effect on the equilibrium HCl concentration at a constant HCl activity (Ag-AgCl buffer) was studied at 600oC, 2 kbar, and KCl and FeCl2 concentrations up to 4m and 1m, respectively. The addition of KCl (up to 4 moles) to the system reduces HCl concentration by 0.4 log units. If hematite or magnetite is present, the quenched HCl concentration drops down to -4 to -4.5 log units.
Annite stability was studied at 600oC and 2 kbar [1]. The decomposition of annite to magnetite and K-feldspar at hydrogen fugacity of 0.4 log units was established with Ag-AgCl sensors. Annite stability in assemblage with K-feldspar, muscovite, and quartz was studied in the presence of Ni-NiO and Co-CoO buffers and over the concentration ranges of KCl 0.3 to 3 m and FeCl2 0.03 to 3 m. The equilibrium ratio mFeCl2/mKCl is constant and equals 0.48+0.2log units within the chloride concentration range covered. The iron solubility in the system magnetite-hematite is five time higher in the presence of 3 m KCl and at a buffered HCl activity.
Reference:
39
- Korzhinskii M.A., Paskal M-L. Roux J. (1992) The international symposium of experimental mineralogy. // Petrology, and Geochemistry. Clermont-Ferrand, France.
Shmulovich K.I., Korzhinskii M.A., Tkachenko S.I., and Bocharnikov R.E. High-temperature fumarole gases of the Kudryavyi Volcano (Iturup Island, Kuril Isles).
The steady high-temperature fumarole activity of the Kudryavyi Volcano (Iturup Island, Kuril Isles) was monitored over the period 1991-1995. The unique macromineralization of rhenium sulfide (ReS2) was discovered in natural sublimates forming at 450-680oC [1]. The detailed studies of geochemical features of the volcanic gases showed their composition to be typical of island-arc volcanoes. According to the isotopic ratios D/H and 16O/18O, the volcanic gases are the mixture of magmatic gases with meteoric waters [2]. The direct measurements of oxygen and sulfur fugacities performed with electrochemical sensors [3] showed that these parameters are dictated by gas reactions between the components of different valence (H2S-SO2). Thus, the volcanic gas is a self-buffering system. The total gas emission was estimated from direct pressure measurements at the top of the volcano as 30.000 ton/day. These data allowed the evaluation of quantities of various components transferring by the gas in a unit of time. Mineralogical and geochemical studies of the original magmatic sublimates precipitated in quartz tubes from the high-temperature gas jets were carried out along with measurement of the temperature gradient. The results obtained allowed the following conclusions:
1. The matter deposition from the magmatic gases under decreasing temperature results in the formation of mineralogical zoning. Compounds of ore elements are deposited in the sequence: oxide 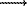 sulfide
sulfide 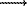 chloride.
chloride.
2. Sublimation in the quartz tubes goes on under conditions close to equilibrium. The temperature decrease due to the mixing of the volcanic gas with meteoric waters is the main factor responsible for the deposition of ore and petrogenic elements.
3. The ore zoning forming as a result of the volcanic gas cooling is similar to the general zoning of hydrothermal deposits.
The precipitation of readily oxidized native metals (Al, Si, Ti, and Fe) from the volcanic gases with redox potential close to Ni-NiO buffer was observed in the quartz tubes that were inserted in the fumarole. A model for this process was proposed, which involves the disproportionation of the low-valence metal compounds in salt melts [4].
References:
- Korzhinskii M.A., Tkachenko S.I., Taran Y.A., Shteinberg G.S., and Shmulovich K.I. (1995) // Nature, vol. 369, no. 6475, pp. 51-52.
- Taran Y.A., Iedenquist J.W., Korzhinskii M.A., Tkachenko S.I., and Shmulovich K.I. (1995) // Geochim. Cosmochim. Acta, vol. 59, no. 9, pp. 1749-1761.
- Osadchii E.G., Lunin S.E., Korzhinskii M.A., and Tkachenko S.I. (1997) // Geokhimiya, no.1, pp. 74-81.
- Korzhinskii M.A., Tkachenko S.I., Shmulovich K.I. and Shteinberg G. (?? ) // Nature, vol. 375, no.6532, p. 544.
Sorokin V.I., Bondarenko G.V. , Gorbaty Yu.E. , Orlov R.Yu. , and Dadze T.P. The experimental study of the S-H2O system by Raman spectroscopy.
The S-H2O system was studied by Raman spectroscopy in the range from room temperatures to 500oC and 1000 bar. This is the pioneering study of this system at such high parameters. H2S, SO2, [HSO4]- , and elementary sulfur were established to be the main solution components. The H2S, SO2, and SO3 (S2O6) concentrations in the gaseous aqueous phase were first determined along the curve of saturated water vapor in the temperature range 180-365oC. The structural studies showed elementary sulfur to be present as S8o form (Bondarenko and Gorbaty, 1995).
The concentrations of dissolved species in the S-H2O system were measured in situ by Raman spectroscopy at the experimental parameters (at 200-365 o C and pressure of saturated H2O vapor). The aqueous phase of the system contains HSO4-, H2S, SO2aq, and Soaq, for which the following temperature dependences were derived (Sorokin et al., 1996):
log mH2S = -2.6969 + 4.5339 ×
10-3T
log mSO2aq = -113069 + 4.7603 ×
10-2T - 5.7204×
10-5 T2,
logmSoaq =-7.4169+ 4.0306×
10-1T-7.3047×
10-5T2 +
+ 1.3152×
10-8T3
The existence of S8o in the aqueous phase of the S-H2O system at temperatures up to 440oC was first proved by structural studies (high-temperature Raman spectroscopy). The concentrations and role of this form at T>200o C are comparable with those of primary species, such as H2S and SO2. The thermodynamic properties of S8o were first estimated. Elementary sulfur was shown to occur in the aqueous phase as eightfold rings with characteristic frequencies equal to 150, 219, and 472 cm-1. The line with the frequency of 237cm-1 in the spectrum indicates the presence of the S7 form. The H2S, SO2, and, presumably, SO3 (S2O6) concentrations in the gaseous phase of the system were measured with a specially designed spectrophotometric cell at 180-312o C and pressures of saturated water vapor. SO3 concentrations in the liquid and gaseous phases are almost identical. H2S concentration in the gaseous phase exceeds that in the liquid, but decreases with increasing temperature (Sorokin and Dadze, 1994, 1995):
log m(H2S)g=-1.38528+0.356519×
103T-1.
Sulfur solubility in the S-H2O system was studied in the temperature range 90-200oC, and the thermodynamic properties of predominant hydrogen polysulfides were first obtained. The incongruent dissolution of hydrogen poly-
40
sulfides in aqueous solutions was shown to be responsible for the redox potential of the H2S-rich heterophase hydrothermal fluids (Migdisov, 1994).
Sretenskaya N.G. and Zakirov I.V. A physicochemical model for gas clusterization.
A model for the gas clusterization (Ar, O2, H2, CO2) under compression was developed and experimentally confirmed in the range of parameters up to the critical density values. The mechanisms and kinetics of the gas clusterization at near-critical parameters were studied with the "Udar" apparatus, which enables recording both rapid (20 msec) and long-term (10 min) pressure and temperature relaxation processes following the shock compression. The system is capable of recording 5000 temperature, pressure, and time values for 100 sec at 20 msec intervals, with accuracies 0.05oC and 0.002 atm, respectively. The compression-expansion experiments with CO2 and Ar at various parameters confirm the cluster mechanism of structural transformations.
According to the experimental data obtained, the deviations from ideal gas in all the above-listed systems can be accurately described by this model within certain pressure and volume intervals. The model is confirmed by the coincidence of the clusterization and condensation heat values for some of the gases and qualitative experimental data on CO2. A set (about 150) of experimental temperature and pressure relaxation curves were obtained for Ar and CO2 at various compression rates and degrees. The pressure relaxation curves for CO2 deviate from the theoretical exponential curve and have a minimum, while those for Ar, which is ideal gas at these parameters, are strictly confined to the theoretical exponential curve.
Reference:
- Zakirov I.V., Sretenskaya N.G., and Chistyakov V.V. Theoretical and experimental molecular clusterization processes at the single-component gas substances, in Experimental and theoretical modeling of the mineral formation processes, Moscow: Nauka (in press).
41
Previous
Contents
Next
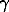 pore (CO2)>1> pore (H2O)] (Zharikov V.A., Shmonov V.M., Shmulovich K.I.). The filtration effect, caused by electrokinetic phenomena or possible component partitioning on heterostatic fluid transport, has affect significantly the transfer of components (Zharikov V.A., Koshemchuk S.K.).
pore (CO2)>1> pore (H2O)] (Zharikov V.A., Shmonov V.M., Shmulovich K.I.). The filtration effect, caused by electrokinetic phenomena or possible component partitioning on heterostatic fluid transport, has affect significantly the transfer of components (Zharikov V.A., Koshemchuk S.K.). 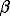 -1,1(int) = -3.5, log
-1,1(int) = -3.5, log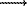 sulfide
sulfide