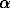 -BN = Li3Mg3B2N5 (1)
-BN = Li3Mg3B2N5 (1)Glass and ceramics properties,
applied mineralogy
Kulinich S.A.., Sevast'yanova L.G., Burdina K. P. and Semenenko K. N. A new lithium-magnesium boronitride: synthesis and properties.
key words [boronitride synthesis]
The data on synthesis and properties of complex ternary nitride compounds of alkaline-alkaline-earth elements and boron are scantily presented in literature. Only lithium-calcium and lithium-barium bo -
52
ronitrides (LiCaBN2 and LiBaBN2, respectively) [1, 2] and LiCa4(BN2)3 obtained by us [3] are presently known.
The objective of this study was to obtain and describe properties of the complex two-metal boronitride in the system Li3N-Mg3N2-BN.
A new complex boronitride of composition Li3Mg3B2N5 (Li3BN2·Mg3BN3) was obtained by sintering in inert gas atmosphere.
The initial reagents were sintered at 950-1000oC for 2-4 hours. The synthesis product was a grey powder relatively stable in air. The sinter obtained was grinded in dry chamber and then repeatedly treated to provide the most complete reaction. The composition of the compound was established by varying the initial component proportions.
If the composition of the sintered mixture is close to LiMgBN2 [1], the reaction proceeds according to
Li3N + Mg3N2 + 2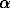 -BN = Li3Mg3B2N5 (1)
-BN = Li3Mg3B2N5 (1)
with an excess of 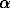 -BN remaining.
-BN remaining.
The new compound was obtained according to the following reactions:
Li3N + Mg3N2 + 2 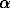 -BN = Li3Mg3B2N5 (1)
-BN = Li3Mg3B2N5 (1)
Li3BN2 + Mg3BN2 = Li3Mg3B2N5 (2)
Li3BN2 + Mg3N2 + 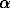 -BN = Li3Mg3B2N5 (3)
-BN = Li3Mg3B2N5 (3)
Li3BN2 + 3Mg + 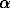 -BN + N2 = Li3Mg3B2N5 (4)
-BN + N2 = Li3Mg3B2N5 (4)
As soon as the composition of the boronitride obtained was determined, a search for optimal synthesis technique was initiated. The method of sintering of the two-metal nitride LiMgN obtained according to [4] with hexagonal boron nitride was tested:
3LiMgN + 2 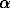 -BN - Li3Mg3B2N5 (5)
-BN - Li3Mg3B2N5 (5)
The lithium-magnesium boronitride obtained from reaction (5) was identical to the products of reactions (1)-(4), judging by X-ray diffractometry data. This fact offered another verification of correctness of determination of the compound composition.
At present, the work on deciphering structure of the newly obtained boronitride is underway. Its behavior at high temperatures and pressures is also being investigated.
References:
Suvorova V.A., Akhmedzhanova G.M., Zyryanov V.N., and Kotel'nikov A.R. Synthesis and properties of iodine-fixing ceramic matrices based on Cu-substituted zeolites.
key words [radioactive iodine immobilization zeolite ceramic disposal]
The immobilization of iodine by Cu-zeolites and sorption of iodine on Ag-zeolites were studied. The possibility of zeolite ceramization was verified. Cu-zeolite was selected for examination, since it is not so expensive as Ag-zeolite, while stability of CuI is comparable with stability of AgI. Industrially produced granulated Cu-zeolite (IONEX, USA) was used in the experiments.
The initial zeolite was annealed at 300oC for 24 h. for dehydration to attain constant weight. The dehydrated zeolite samples together with the excess of crystalline iodine were held in evacuated glass capsules at 130oC for 12-24 h. for iodine saturation. After the runs the zeolite grains were washed in ethanol and chloroform in tandem to remove the excess of iodine. CuI reflexes were not found at the X-ray patterns of the samples.
Another method of introduction of iodine into zeolite consisted in synthesis of ceramics from the dehydrated initial Cu-zeolite in the presence of excess of iodine and water (1 wt %) in hermetically sealed platinum capsules at 500oC and 1000 atm. Quenched samples appeared as dense cylinders 30-40 mm long and 3-5 mm in diameter. The X-ray patterns of the samples included CuI reflexes and reflexes probably corresponding to aluminosilicates, such as andalusite, mullite, etc. The atomic ratio of iodine to copper ranged from 0.05 to 0.84, i. e. only a part of the copper atoms proved to be capable of combining with iodine. The ceramics samples obtained in these runs had density of 2.5 - 2.7 g/cm3 and open porosity 0 - 0.53%.
The third experimental series involved introduction of iodine into the zeolite samples. Copper in zeolites was preliminarily reduced as follows. Spectral pure graphite was placed to the bottom of a quartz capsule, and the dehydrated zeolite grains were placed in the middle of the capsule on the detachable screen made of perforated nickel. The cap -
53
sules were heated in cylindrical resistance furnaces that provided vertical temperature gradient from 750 -780oC at the bottom to 280-500oC at the screen. The copper reduction (copper became gray-rose) occurred through the interaction with CO, which forms when graphite is heated above 700oC.
Saturation of the samples with iodine was carried out as described above. The X-ray diffraction patterns of the samples showed clear reflexes of CuI and the starting zeolite.
Iodine-saturated zeolites were dried for two days at 100oC and then subjected to ceramization in sealed platinum capsules under conditions of hot isostatic pressing at 800oC and 1000 atm. Ceramics samples (cylinders) had density of 2.3 - 2.5 g/cm3 and porosity 13 - 22%. The Cu/I ratio exceeding unity indicated that iodine was completely bound by copper. The ceramics quality was evaluated from leachability of elements from the samples in distilled water at 90oC. The results of the leaching experiments are presented in figure.
From the results obtained, we made the following conclusions:
1. Cu-zeolite does not bind iodine, when these components are mixed in hermetically sealed vessel at 130oC for 24 h.
2. Cu-zeolite binds iodine as a result of ceramization of zeolite-iodine mixtures under hydrothermal conditions. The leach rate reaches its maximum for the first eight days for all the samples. For this period, 4% of iodine of the sample passes to the leaching solution; after that the leach rate averages 0.93 g/m2×day, and the concentrations of leached iodine, silica, and copper are 1-2, 0.1, and >0.1%, respectively. After 36 days the average leach rate for SiO2 is 0.04 g/m2×day, and for copper - 0.06 g/m2×day.
3. Iodine is well bound by Cu-zeolite, providing the copper of the zeolite was preliminarily reduced by ceramization in gasostat. The ceramics density is close to that of the hydrothermal samples, however, the porosity is very high. Nevertheless, the average iodine leach rate decreases to 0.7 by the 22nd day (lgV = -0.17) g/m2×day. Unreduced zeolite shows similar value for iodine, but higher values for silica and copper (0.13 and 1.3 respectively). Ceramization of granulated zeolite evidently yields very porous samples. They readily disintegrate and lose their main components, such as copper, which binds iodine. Thus, the iodine leach rate did not decrease, as was expected from high values of the I/Cu ratio.
Leaching experiments with iodine-bearing ceramics, based on Cu-zeolite, showed their high stability. After 36 days the average iodine leach rate was 0.9 g/m2× day, which is 4-5 times lower than the rate of iodine leaching from sodalite (Shidlovsky et al.) [1] and comparable with leach rates of alkaline and alkali-earth elements from borosilicate glasses [2].
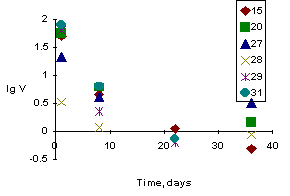
Figure. Kinetics of iodine leaching from ceramics samples based on Cu-zeolite.
Thus, there are several benefits of such a method of iodine immobilization. The material obtained is rather stable and provides low-volume matrix capable of absorbing radioactive iodine directly from the gaseous jets produced by nuclear plants. The marked tendency of decrease of leach rate (by a factor of 20-50 for 36 days) and leaching percentage (by a factor of 4-5 for a week) suggests that the percentage of iodine leaching from these samples will not subsequently exceed 0.5 and become negligible in long-term storage.
References:
54