Radioactive wastes
Suvorova V.A., Zyryanov V. N., Akhmedzhanova G.M., Kotel'nikov A.R., and Tikhomirova V.I. Study of possible combining radioactive iodine by ceramic matrixes based on NaX zeolite.
key words [zeolite radioactive iodine]
Combining iodine by NaX-zeolite was studied to verify the feasibility of synthesis of zeolite ceramics saturated with iodine. The industrially prepared NaX-zeolite (GOZ VNIIP) of
Na2O*Al2O3*2.5SiO3*6H2O composition
was chosen for the studies for its high (63%) sorption capacity relative to iodine [1] and capability of yielding ceramics with stable sodalite structure.
The initial zeolite was preliminarily dried at 110oC and annealed at 300oC during 24 h for dehydration to attain constant weight. The dehydrated zeolite samples together with the excess of crystalline iodine were held in evacuated glass capsules at 130-400oC during 12-24 h. for iodine saturation. After the runs the zeolite grains were washed in ethanol and chloroform in tandem to remove the excess of iodine. NaI reflexes were not found at the X-ray patterns of the samples.
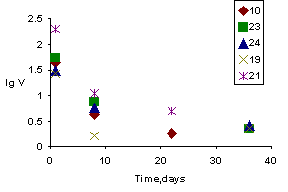
Another method of introduction of iodine into zeolite consisted in synthesis of ceramics from the dehydrated initial zeolite in the presence of iodine excess and water (1 wt %) in hermetically sealed platinum capsules at 500oC and 1000 atm. Quenched samples appeared as dense cylinders 30-40 mm long and 3-5 mm in diameter. The X-ray patterns of the samples comprised relatively weak reflexes of NaI, reflexes of the phase similar to iodine-free zeolite ceramics, and the new phase of composition corresponding to stoichiometric iodosodalite synthesized at 700oC and 1 atm [2]. The maximum atomic ratio of iodine to sodium was 0.3, that is only a part of sodium atoms proved to be capable of combining with iodine. The ceramic samples obtained in these runs had density of 2.4 g/cm3 and open porosity 1.4 - 3.3%. The ceramics quality was evaluated by the leaching elements from the samples in distilled water at 90oC (test MAGATE MCC-1). The results of the studies are presented in table (Runs 10, 23, and 24).
The third experimental series involved introduction of iodine into the samples by ceramization of the starting NaX-zeolite with dry copper iodide (20 wt %) at 500 C and 1000 atm in platinum capsules using vessels with cold lock or at 1000oC and 1000 atm in gasostats. The run products had density of 2.4 - 2.6 g/cm3 and porosity 0 - 1.1%. The X-ray patterns of the samples included reflexes of CuI, ceramic matrix, and a new phase which was similar in reflection parameters to copper NaX-zeolite. The absence of NaI reflexes and low (< 0.1) I/Na ratios suggest that no active interaction between sodium and iodine occurred. The Cu/I ratio equal 1 - 1.9 indicates that iodine was completely bounded by copper (table, Samples 19 and 21).
From the results obtained, we made the following conclusions:
1. The NaX-zeolite does not binds iodine on heating in hermetically sealed vessel within the temperature range 130- - 400oC during 24 h.
2. The NaX-zeolite combines iodine as a result of ceramization of zeolite-iodine mixtures at 500oC and 1000 atm. The leach rate reaches its maximum for the first eight days for all the samples. For this period, 3 - 5% of iodine of the sample passes to the leaching solution. After eight days, the leach rate averages 2 g/m2 * day, and the concentrations of leached iodine, silica, and sodium are 2 - 4, 0.13, and 1 - 2%, respectively. High leach rates for SiO2 (0.1 g/m2 * day) and Na (1 g/m2 * day) after 36 days point to the low stability of this ceramics (including the blank ceramics, Run 8).
3. Iodine is loosely bound by the NaX-zeolite mixed with dry CuI and subjected to hot isostatic pressing at 1000oC. The iodine leach rate remains equal 2 - 4 (lgV = 0.3 - 0.6) g/m 2 * day by the twenty second day; those for silica and sodium are relatively high: 0.13 (lgV = -1) and 1 (lgV = 0.1), respectively. The leached element concentrations are also high. Evidently, iodine is not retained by the ceramic matrix because of low NaX-zeolite stability which is unaffected even by the copper iodide admixture.

Fig. Leach rate of iodine from ceramic samples based on zeolite NaX; in legend indicated numbers of runs.
55
Table. Leach rates V (g/m2 * day) of elements from the samples obtained by ceramization of the mixtures of NaX-zeolite with iodine and CuI
|
Sample no |
Element |
Conc. wt %. |
d,g/cm3 |
% |
Run duration, day |
|||
|
0-1 |
1-7 |
7-14 |
7-28 |
|||||
|
8 |
SiO2 |
50.05 |
2.34 |
|||||
|
Na |
9.92 |
8.2 |
3.1 |
2.2 |
0.124 |
- |
||
|
10 |
I |
14.00 |
- |
83.3 |
2.5 |
1.30 |
- |
|
|
SiO2 |
41.50 |
- |
43.7 |
4.2 |
1.83 |
- |
||
|
I |
9.10 |
2.42 |
4.0 |
2.2 |
0.71 |
- |
||
|
23 |
SiO2 |
43.40 |
3.3 |
54.9 |
7.6 |
- |
2.20 |
|
|
Na |
12.10 |
4.2 |
0.5 |
- |
0.09 |
|||
|
I |
8.56 |
2.45 |
13.9 |
3.1 |
- |
0.76 |
||
|
24 |
SiO2 |
43.70 |
1.4 |
30.7 |
5.8 |
- |
2.62 |
|
|
Na |
9.64 |
2.9 |
0.8 |
- |
0.09 |
|||
|
19 |
I |
1.51 |
2.48 |
22.9 |
4.4 |
- |
1.37 |
|
|
SiO2 |
51.58 |
0.0 |
26.9 |
1.7 |
- |
2.30 |
||
|
21 |
I |
2.64 |
2.62 |
1.0 |
0.1 |
- |
0.04 |
|
|
SiO2 |
47.63 |
1.1 |
193.0 |
11.2 |
4.70 |
- |
||
|
Na |
10.24 |
0.2 |
0.1 |
0.12 |
- |
|||
|
Cu |
0.93 |
24.4 |
6.1 |
1.35 |
- |
|||
|
0.5 |
0.1 |
0.06 |
- |
|||||
The experiments with iodine-bearing ceramics based on NaX-zeolite showed that for 36 days the iodine leach rate averages 2.2 (lgV = 0.35) g/m2 * day, half as high as than that for the sodalite-like material [3] and comparable with the leach rates of alkaline and alkali-earth elements from borosilicate glasses [4]. Therefore, we can expect the long retention of iodine in these matrixes.
References:
56