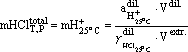 (1)
(1)Solubility of baddeleyite (ZrO2) and zircon (ZrSiO4)
in aqueous hydrochloric solutions at elevated T and P parameters
Korzhinskaya V.S.
Institute of Experimental Mineralogy
Russian Academy of Sciences 142432 Chernogolovka Moscow distr., Russia
#
The work has been sponsored by the RFBR (Grant N 97-05-65826, 99-05-64106, Grant of the leading research schools 96-16-98333)Information on the stability of zirconium compounds is very important for understanding geochemical behaviour of such minerals as baddeleyite and zircon in hydrothermal processes. It is still to be answered why zircon is uncommon to hydrothermal conditions of formation of rare metal and other deposits which are accompanied by considerable wall rock alterations. As is known, zircon is found only in rocks associated with alkaline metasomatism. The problem can be tackled through experimental studies on solubility of zircon and baddeleyite, on the dependence of their stability on temperature, pressure, pH and composition of the active solution under hydrothermal conditions.
Presently, the information on the behaviour of zirconium in solutions at elevated T-P parameters is scarce, and the available data obtained for water solutions pertain to normal conditions. Due to high charge, small radius and comparatively small ionisation potential zirconium ions in a solution tend to hydrolysis and complexing with anions of organic and inorganic acids. Therefore, because of complexity and peculiarity of chemical behaviour of zirconium compounds in water solutions the problem of forms of existence of ions in them has not been conclusively solved [36-37].
According to Nazarenko et. al.(1979) and Savin et. al.(1974) [26, 33] such hydrocomplexes of zirconium as Zr+4 , Zr(OH)+3, Zr(OH)2+2, Zr(OH)3+, Zr(OH)4 exist, depending on pH, in a solution under normal conditions. The system ZrO2-HNO3-H2O was studied by Sazhina et. al., (1982) at T=25oC in the range of nitric acid concentration 10.8-53.7 wt % and 26.3-9.18 wt % ZrO2 [31]. It was shown that under those conditions the concentration of zirconium in a nitric acid solution decreased with growing acid concentration. A minimum solubility value was in the range of 45-50 wt pct. HNO3.
Karlysheva et. al.(1982 ) [13] studied zirconium complexing with CO3-ions . They investigated the interaction of zirconium oxychloride (ZrOCl2) with Na2CO3 in water solutions using IR spectroscopy and pH-metry to find that a Zr(OH)2(CO3)2-2 complex forms at pH=7. The complex decomposed with decreasing pH to form Zr(OH)4 at pH=1.8. At greater length the complexing of zirconium in hydrocarbonate solutions was studied by Samchuk and Dorofey (1983 ) [32] for the system Zr(OH)4-HCO3-H2O at 25oC. The authors showed that the presence of hydrocarbonate-ions increased the solubility of Zr(OH)4 by 2-3 orders of magnitude as compared with water. It was concluded that hydrobicarbonate complexes were one of the forms of zirconium migration in natural waters.
The problem of the influence of physicochemical mineral formation conditions on the deposition of zircon and baddeleyite in ore bodies has not, however, been solved because some thermodynamic constants of these two minerals and of zirconium particles in a hydrothermal fluid are unknown or unreliable. Therefore, direct experimental studies of mineral solubility at elevated T-P parameters are of primary importance.
In this report we give the results of a study of baddeleyite and zircon solubility in a supercritical HCl fluid in a broad range of temperatures and concentrations.
Methods and experimental techniques
Solubility studies of zirconium minerals were performed by capsule and buffer techniques.
Solubility of baddeleyite in a HCl fluid was studied by a capsule technique with a further analysis of the composition of quenched solution and solid phase in a hydrothermal high pressure apparatus [12]. The experimental temperatures ranged from 400 to 600oC, pressures from 1 to 2 kbar, muriatic acid concentrations from 10-2 to 4 mole/kg H2O. The measurement accuracy was not lower than +5oC for the temperature and +25 bar for the pressure. The runs were conducted in platinum tubes (10x0.2x50 mm) whereinto a mineral charge (30mg) was placed. Both chemical reagent ZrO2 (high purity grade) in the form of crystalline substance (crystal sizes 15-30 μm) and synthetic baddeleyite grown by us under hydrothermal conditions at T=400-500oC and P=1 kbar from ZrCl4 were used as the mineral charge. Muriatic acid solutions of preset concentration were prepared from ficsanal? by diluting them with bidistilled water. HCl concentrated solutions were prepared from HCl conc. (high purity grade).
The runs concerned with the effect of salt loading (NaCl) on the solubility of baddeleyite were carried out at T=500oC, P=1 kbar and HCl concentrations: 0.01; 0.1, and 1 mole/kg H2O. The concentration of NaCl was 0.1; 0.5; 1 and 2 mole/ kg H2O. The amount of the solution in capsules was constant ( 1ml ).
The charged capsule was sealed and held under static conditions at chosen T-P. After quenching the capsule was opened and its contents was washed out with distilled water, acidified with HCl, into a test tube. The solution was then separated from the mineral charge with the aid of a filter. Zirconium in the solution was determined photocalorimetrically at an apparatus SPECOL using an arsenazo III indicator [1]. Inasmuch as an equilibrium concentration of zirconium in the solution was always much lower than the concentration of hydrochloric acid, the values of the latter after the run were taken to be equal to its values before the run. The composition of solid products were examined in a polarizing microscope and by X-ray diffraction. Always, after the run , baddeleyite was the only solid phase.
9
Baddeleyite reached an equilibrium after 5-14 days which was found by special runs of different durations for T=400, 50, 600oC, P=1 kbar, and 1m HCl. As seen from the diagram in fig.1, for 400oC in the beginning, in the first 2-4 days, the concentration of zirconium in the fluid becomes greater that the equilibrium value, and it gradually decreases down to the equilibrium value in the course of 10-14 days. This phenomenon is observed in studies of other minerals, from example in the works of Korzhinskiy (1987), Kudrin (1985) [17, 19]. It can be attributed to different rates of the direct and reverse reactions of dissolution and precipitation.
We studied the solubility of zircon in the system ZrO2-SiO2-H2O-HCl in the temperature range 400-600oC, at P=1 kbar and HCl concentrations from 1.8 to 0.02 mole/kg H2O using a buffer technique enabling the maintenance of HÑlo constant activity at given T and P conditions [6]. The buffer was a solid Ag-AgCl mixture in combination with various redox buffer (Ni-NiO; Co-CoO; Fe3O4-Fe2O3). In our runs we used a modified method enabling us to approach an equilibrium from two sides [15]. Fig.2 represents schematically the charging of capsules. The procedure was as follows: four platinum capsules measuring 3x0.1x50 mm and 4.0.1.40 mm were located in the same working volume of constant T, P, and chemical potential of hydrogen. Two of the capsules (3 mm) acted as fixing agents of HClo activity and contained a solid buffering mixture Ag-AgCl and a H2O-HCl solution of various initial concentrations. The two other capsules (4 mm) contained the same Ag-AgCl buffer, zircon-quartz association and ZrCl4aq of various initial concentrations. The hermetically sealed platinum capsules were located in a large gold capsule (10x0.2x50 mm) together with an oxygen solid buffer. There into a required amount of distilled water was filled after which the gold capsule was hermetically sealed and placed into a hydrothermal vessel to perform a run at the given T and P parameters until an equilibrium was reached. The duration of the runs varied from 10 days for 600oC to 30 days for 400oC [3, 8, 16]. Such a procedure made it possible to control simultaneously in all the four platinum capsules the hydrogen fugacity and, accordingly, an equilibrium concentration of HClo, an in the capsules, containing the zircon + quartz charge, an equilibrium concentration of Zraq at the given T,P parameters. The equilibrium in the system was considered to be reached when the concentration of HClo in the two platinum capsules, containing only the Ag-AgCl buffer, differed by no greater than +0.06 log.un., and the concentrations of zirconium in the fluid in the other two capsules differed by no greater than +0.07 log.un. mZraq. After the run the concentration of HCl was defined from the pH value of the quenched solution taken from the capsules containing only the Ag-AgCl buffer. To that purpose 0.1 ml quenched solution was taken with a calibrated pipet (0.1 + 0.0005 ml) and diluted with bidistilled water (1 ml). The measured pH value was recalculated using the following equilibrium:
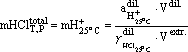 (1)
(1)
were Vdil., aH+25oCdil., g HCl25oCdil., are the volume, the hydrogen ion activity, and the coefficient of HCl activity, respectively, pertinent to the diluted solution [2]; Vextr. is the volume of the extracted quenched solution. The concentration of zirconium in the solution from the other two capsules was measured photocalorimetrically with arsenazo III.
Fig.1. Charging of capsules, schematic. 1 - sealed gold capsules; 2-3 - platinum capsules with buffers Ag-AgCl+H2O and Ag-AgCl+HCl+H2O; 4-5 - platinum capsules with buffers Ag-AgCl + Zrc+Q+H2O and Ag-AgCl+Zrc+Q+ZrCl4aq; 6 - MOx-MOy (redox couple) buffer.
Fig.2. Results of kinetic runs in the system ZrO2-H2O-HCl at T=400-600oC and P=1 kbar (mHCl=1 mole/kg H2O)
10
Table 1. Conditions and results of the baddeleyite solubility study in the system ZrO2-H2O-HCl ( capsule technique)
Sample number |
mHCl mole/kg H2O |
lgmHCl |
aH+ |
+γ |
-lgKdHClo |
mHClo mole/kg H2O |
-lgmHClo |
-lgmZraq |
T=400oC P=1kbar t=14d VH2O=1.443 |
||||||||
1 |
0.01 |
2.000 |
5.91E-03 |
0.7946 |
-1.867 |
2.57E-03 |
2.590 |
4.074 |
2 |
0.1 |
1.000 |
4.38E-02 |
0.6298 |
5.62E-02 |
1.251 |
4.012 |
|
3 |
0.2 |
0.699 |
4.17E-02 |
0.5783 |
0.128 |
0.893 |
3.885 |
|
4 |
0.4 |
0.398 |
6.20E-02 |
0.5285 |
0.283 |
0.549 |
4.068 |
|
5 |
0.65 |
0.187 |
8.12E-02 |
0.4953 |
0.486 |
0.313 |
4.022 |
|
6 |
0.8 |
9.69E-02 |
9.11E-02 |
0.4817 |
0.611 |
0.214 |
3.975 |
|
7 |
1.0 |
0 |
0.1029 |
0.4675 |
0.780 |
0.108 |
3.683 |
|
8 |
2.0 |
-0.301 |
0.1496 |
0.4263 |
1.649 |
-0.217 |
2.157 |
|
9 |
4.0 |
-0.602 |
0.2163 |
0.3901 |
3.446 |
-0.537 |
0.969 |
|
10 |
H20 |
4.890 |
||||||
T=400oC P=2kbar t=14d VH2O=1.261 |
||||||||
1 |
0.01 |
2.000 |
7.64E-03 |
0.8172 |
-1.052 |
6.54E-04 |
3.183 |
3.990 |
2 |
0.1 |
1.000 |
4.77E-02 |
0.6416 |
2.56E-02 |
1.591 |
4.050 |
|
3 |
0.2 |
0.699 |
7.77E-02 |
0.5880 |
6.79E-02 |
1.168 |
3.955 |
|
4 |
0.4 |
0.398 |
0.1233 |
0.5386 |
0.171 |
0.767 |
4.420 |
|
5 |
0.65 |
0.187 |
0.1682 |
0.5072 |
0.318 |
0.497 |
3.830 |
|
6 |
0.8 |
9.69E-02 |
0.1915 |
0.4947 |
0.413 |
0.384 |
3.720 |
|
7 |
1.0 |
0 |
0.2198 |
0.4819 |
0.544 |
0.265 |
3.728 |
|
8 |
2.0 |
-0.301 |
0.3335 |
0.4460 |
1.252 |
-0.098 |
2.030 |
|
9 |
4.0 |
-0.602 |
0.4987 |
0.4161 |
2.801 |
-0.447 |
0.811 |
|
T=500oC P=1kbar t=7d VH2O=1.892 |
||||||||
1 |
0.01 |
2.000 |
7.18E-04 |
0.8765 |
4.251 |
9.18E-03 |
2.037 |
4.712 |
2 |
0.1 |
1.000 |
2.33E-03 |
0.7877 |
9.70E-02 |
1.013 |
4.583 |
|
3 |
0.2 |
0.699 |
3.31E-03 |
0.7524 |
0.196 |
0.709 |
4.542 |
|
4 |
0.4 |
0.398 |
4.70E-03 |
0.7125 |
0.393 |
0.405 |
4.375 |
|
5 |
0.65 |
0.187 |
6.00E-03 |
0.6819 |
0.641 |
0.193 |
4.710 |
|
6 |
0.8 |
9.69E-02 |
6.70E-03 |
0.6681 |
0.790 |
0.102 |
4.171 |
|
7 |
1.0 |
0 |
7.40E-03 |
0.6529 |
0.988 |
0.005 |
4.112 |
|
8 |
2.0 |
-0.301 |
1.06E-02 |
0.6030 |
1.982 |
-0.297 |
3.855 |
|
9 |
4.0 |
-0.602 |
1.49E-02 |
0.5496 |
3.973 |
-0.599 |
2.759 |
|
10 |
H20 |
5.430 |
||||||
T=500oC P=2kbar t=7d VH2O=1.446 |
||||||||
1 |
0.01 |
2.000 |
3.70E-03 |
0.8319 |
2.624 |
5.61E-03 |
2.251 |
4.618 |
2 |
0.1 |
1.000 |
1.38E-02 |
0.7089 |
8.10E-02 |
1.094 |
4.631 |
|
3 |
0.2 |
0.699 |
2.01E-02 |
0.6661 |
0.170 |
0.770 |
4.420 |
|
4 |
0.4 |
0.398 |
2.90E-02 |
0.6215 |
0.353 |
0.452 |
4.367 |
|
5 |
0.65 |
0.187 |
3.73E-02 |
0.5897 |
0.587 |
0.232 |
4.657 |
|
6 |
0.8 |
9.69E-02 |
4.16E-02 |
0.5760 |
0.728 |
0.138 |
3.955 |
|
7 |
1.0 |
0 |
4.67E-02 |
0.5614 |
0.917 |
0.038 |
4.061 |
|
8 |
2.0 |
-0.301 |
6.67E-02 |
0.5167 |
1.871 |
-0.272 |
2.822 |
|
9 |
4.0 |
-0.602 |
9.50E-02 |
0.4739 |
3.799 |
-0.580 |
1.953 |
|
T=600oC P=1kbar t=7d VH2O=2.673 |
||||||||
1 |
0.01 |
2.000 |
3.48E-05 |
0.9531 |
6.915 |
9.96E-03 |
2.002 |
4.656 |
2 |
0.1 |
1.000 |
1.10E-04 |
0.9173 |
9.99E-02 |
1.001 |
4.486 |
|
3 |
0.2 |
0.699 |
1.56E-04 |
0.9021 |
0.200 |
0.699 |
4.502 |
|
4 |
0.4 |
0.398 |
2.20E-04 |
0.8842 |
0.400 |
0.398 |
4.520 |
|
5 |
0.65 |
0.187 |
2.81E-04 |
0.8699 |
0.650 |
0.187 |
4.395 |
|
6 |
0.8 |
0.097 |
3.12E-04 |
0.8632 |
0.800 |
0.097 |
4.310 |
|
7 |
1.0 |
0 |
3.49E-04 |
0.8557 |
0.99996 |
0 |
4.301 |
|
8 |
2.0 |
-0.301 |
4.93E-04 |
0.8298 |
1.9994 |
-0.301 |
4.063 |
|
9 |
4.0 |
-0.602 |
6.97E-04 |
0.7997 |
3.9991 |
-0.602 |
2.968 |
|
10 |
H20 |
5.050 |
||||||
T=600oC P=2kbar t=7d VH2O=1.694 |
||||||||
1 |
0.01 |
2.000 |
7.97E-04 |
0.8927 |
4.156 |
9.11E-03 |
2.041 |
4.881 |
2 |
0.1 |
1.000 |
2.60E-03 |
0.8154 |
9.68E-02 |
1.014 |
4.827 |
|
3 |
0.2 |
0.699 |
3.69E-03 |
0.7847 |
0.195 |
0.709 |
4.799 |
|
4 |
0.4 |
0.398 |
5.24E-03 |
0.7500 |
0.393 |
0.406 |
4.739 |
|
5 |
0.65 |
0.187 |
6.69E-03 |
0.7232 |
0.641 |
0.193 |
4.604 |
|
6 |
0.8 |
0.097 |
7.43E-03 |
0.7112 |
0.789 |
0.103 |
4.517 |
|
7 |
1.0 |
0 |
8.31E-03 |
0.6979 |
0.988 |
5.21E-03 |
4.357 |
|
8 |
2.0 |
-0.301 |
1.18E-02 |
0.6542 |
1.982 |
-0.297 |
3.162 |
|
9 |
4.0 |
-0.602 |
1.67E-02 |
0.6071 |
3.973 |
-0.599 |
2.321 |
|
11
The zircon-quartz association is a mixture of zircon and quartz minerals (40 mg). We used synthetic quartz furnace-preheated to 900oC and cooled in cold distilled water. The monolith cracked to pieces which were ground to powder (30 μm particles) in an agate mortar. Synthetic ZrSiO4 of tetragonal syngony with the grain size of 10-15 μm, produced by us under hydrothermal conditions, was used as the mineral zircon [11].
Results and discussion
Solubility of baddeleyite. The experimental results on the baddeleyite solubility in the system ZrO2-H2O-HCl are given in table 1. The bulk concentrations of HCl in the fluid after the run were recalculated with respect to the concentration of the associated HClo using the data on the dissociation constants of hydrochloric acid, borrowed from the work by Frantz and Marshall (1984) [7]. The activity coefficients for ionic particles were calculated from the second-order Debye-Huckel equation [10].
Figs. 3a,3b gives the logarithmic dependence between the concentration of the associated HClo and the concentration of zirconium in equilibrium with baddeleyite for temperatures 400, 500, and 600oC and pressure P = 1kbar (fig.3a) and 2 kbar (fig.3b). All the isotherms demonstrate the same picture, namely, their slopes do not remain constant as the concentration of hydrochloric acid grows. In water and in the region of low HCl concentrations (HCl concentration Ð 0.2 m ) the solubility of baddeleyite is, practically, independent of the HClo concentration. The dominant equilibrium in this region, like in water, is believed to be as follows:
ZrO2 + 2H2O = Zr(OH)4o (2)
with the equilibrium constant
Kp = mZr(OH)4o (3)
The solubility of baddeleyite is insignificant in this region and, as seen from table 1, makes up: for 400o - 1.28.10-5, for 500o - 3.72.10-6, and for 600oC 8.90.10-6 mole/kg H2O.
The general principles of experimental data treatment aimed at identification of particles, formed in the solution, involve the comparison of theoretically expected slopes of solubility isotherms as a function of chlorides concentration from a number of hypothetic reactions of mineral dissolution with experimentally established slopes, and a further selection of reactions in accordance with the criterion of best fit of the theoretical and experimental slopes. In this scheme it is supposed that associated neutral particles dominate in the solution because the study was carried out at high temperatures and using high concentrations of hydrochloric acid. Based on this method and using the information Mwakio (1985), Nazarenko et.al.(1979) [23, 26], on the state of zirconium ions in water solutions at 25o and 80oC , one can suppose that at T=400-600oC and P=1 kbar Zr(OH)4o, Zr(OH)3Clo, Zr(OH)2Cl2o, Zr(OH)Cl3o, and ZrCl4o were dominant particles in the solution.
In the range of hydrochloric acid concentrations 0,2-0,8 mole/kg H2O the slope of isotherms which reflect the relationship between the associated HClo and Zraq , dissolved in the fluid, is not constant. This may be associated with a change of zirconium complexes in the solution simultaneously. The following equilibria can exist, theoretically, in this region
ZrO2 + HClo = Zr(OH)3Clo (4)
ZrO2 + 2HClo = Zr(OH)2Cl2o (5)
ZrO2 + 3HClo = Zr(OH)Cl3o (6)
Their equilibrium constants have the form:
Kp = mZr(OH)3Clo/mHClo (7)
Kp = mZr(OH)2Cl2o/m2HClo (8)
Kp = mZr(OH)Cl3o/m3HClo (9)
In the region of concentrated hydrochloric solution (mHCl³ 0,8 mole/kg H2O) the solubility of baddeleyite drastically increases by 2-4 orders of magnitude. In this region (dlog mZraq/dlog mHCl)=4 and the dominant equilibrium in this region will be the following one
ZrO2 + 4HClo = ZrCl4o + 2H2O (10)
with the equilibrium constant:
Kp = mZrCl4o/m4HClo (11)
Table 2 giver the calculated values of the equilibrium constants (2,4,5,6,10).
Comparison of the baddeleyite solubility data obtained at different temperatures and constant pressure and hydrochloric acid concentration shows that the concentration of zirconium in the solution decreases with growing temperature. For example, at P=2 kbar for the diluted region (mHCl=0,01 m) the solubility of baddeleyite is 8.91.10-5 at 400o; 2.34.10-5 at 500o, and 1.49.10-5 at 600oC. In the concentrated region (mHCl=4 m) the concentration of zirconium in the solution is 1.55.10-1 at 400o; 1.11.10-2 at 500o; and 1.78.10-3 at 600oC. It should be mentioned that such a behavior is observable for other chloride salts as well in studies of hydrolysis reactions [8,9,28].
12
Table 2. Equilibrium constants of the reactions, controlling the baddeleyite solubility
Reaction |
-lgKp |
||
400o |
500o |
600o |
|
ZrO2 + 2H2O =Zr(OH)4o |
4.89+0.120 |
5.43+0.025 |
5.05+0.14 |
ZrO2 + HClo + H2O = Zr(OH)3Clo |
3.60+0.021 |
4.16+0.070 |
4.30+0.061 |
ZrO2 + 2HClo =Zr(OH)2Cl2o |
3.48+0.033 |
4.40+0.042 |
4.61+0.045 |
ZrO2 + 3HClo =Zr(OH)Cl3o |
3.39+0.012 |
4.76+0.019 |
5.00+0.031 |
ZrO2 + 4HClo =ZrCl4o+ H2O |
3.32+0.017 |
5.16+0.025 |
5.48+0.030 |
However, as seen from fig.3a, at P=1 kbar, this dependence in the diluted region of HCl concentrations is different, and an equilibrium concentration of zirconium is higher at T=600oC than at T=500oC. At mHCl = 0.01 m the concentration of zirconium in the solution is 1.94.10-5 at 500oC and 2.21.10-5 mole/kg H2O at 600oC. A pressure growth from 1 to 2 kbar, temperature and HCl concentration being constant, leads to an increase of mZraq. The role of pressure is, however, less significant.
Fig.3. (a,b) - Solubility of baddeleyite in HCl solutions at T=400-600oC.
Table 3. Conditions and results of the baddeleyite solubility study in the system ZrO2-H2O-HCl (technique involving a Ag-AgCl buffer)
Initial solution |
pH |
-lgmHCl total |
mHClo mol/kg H2O |
-lgmHClo |
-lgmZraq |
-lgH+ |
+γ |
aH+ |
T=500oC P=1kbar t=15d VH2O=1.892 Co-CoO-Ag-AgCl-H2O-HCl
H2O |
1.34 |
0.08 |
0.8215 |
0.0854 |
- |
1.991 |
0.6655 |
6.79E-03 |
1.5mHCl |
1.33 |
0.09 |
0.8028 |
0.0954 |
- |
1.997 |
0.6671 |
6.71E-03 |
H2O |
- |
- |
3.990 |
|||||
0.1mZrCl4 |
- |
- |
4.069 |
T=500oC P=1kbar t=15d VH2O=1.892 Ni-NiO-Ag-AgO-H2O-HCl
H2O |
1.61 |
0.33 |
0.4552 |
0.3418 |
- |
2.143 |
0.7036 |
5.06E-03 |
1mHCl |
1.60 |
0.34 |
0.4499 |
0.3468 |
- |
2.147 |
0.7043 |
5.03E-03 |
H2O |
- |
- |
4.362 |
|||||
0.1mZrCl4 |
4.296 |
In fig.3a (shaded squares) and table 3 are given the data on the solubility of baddeleyite at T=500oC and 1 kbar obtained with the use of Ag-AgCl buffer in combination with oxygen buffers Co-CoO and Ni-NiO. As seen from fig.3a the solubility values of baddeleyite obtained by the capsule technique and by using buffer are in good agreement, the differences do not exceed 0.05 log un.
13
Fig.4 illustrates the results of three series of runs on baddeleyite solubility in mixed HCl+NaCl solutions at T=500oC and P=1 kbar. As seen from the diagram, with the initial HCl concentration of 0,1 and 0,01m the concentration of zirconium in the solution is lower throughout than in the absence of electrolyte. This may be attributed to partial dissociation of hydroxochloride complexes of zirconium due to addition into the solution of extra chlorine ion that decreases an equilibrium zirconium concentration. At mHCl=1 mole/kg H2O the adding of NaCl increases the solubility of baddeleyite that is attributed to the formation of undissociated complex of zirconium with sodium. Analogous results were obtained in cassiterite solubility studies at the same T and P parameters in HCl+NaCl solutions performed by Kovalenko et. al. (1986) [18].
Fig.4. Solubility of baddeleyite in HCl+NaCl solutions at T=500oC and P=1 kbar.
Fig.5. Solubility of zircon in a water solution of HCl at 400, 500, 600oC and P=1 kbar in the presence of quartz.
Fig.6. Concentrations of zirconium and silicon in a solution in equilibrium with baddeleyite-zircon and zircon-quartz associations at T=600oC, P=1 kbar, mHCl=1 mole/kg H2O. 1 - experimental data; 2 – calculations from thermodynamic data.
Fig.7. Alterations of the amount of equilibrium SiO2aq in a solution vs the temperature for a baddeleyite-zircon association.
Solubility of zircon. Incongruent solubility of zircon with the formation of quartz can be represented in the form of equilibrium:
ZrSiO4+ xHCloaq= SiO2 + Zr(OH)4-nClnoaq + (x-2) H2O
Zrc Q (12)
The equilibrium constant of this reaction is as follows:
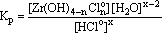 (13)
(13)
Under the assumption that the behavior of uncharged particles in the fluid is ideal and the activity of water is close to unity, this constant can be written as
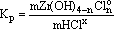 (14)
(14)
where m is the molality (mole/kg H2O). As suggested by the equilibrium (14), the solubility of zircon depends solely on the activity of associated HCl in the solution at given T,P conditions. By analogy with the baddeleyite solubility study, the gross HCl concentration values were recalculated with respect to the concentration of undissociated HClo, and zirconium dissolved in the fluid phase was in the associated forms.
14
The initial conditions and the experimental results are given in table 4. Fig.5 shows the relationship between the concentration of zirconium in hydrothermal solution and the concentration of associated HClo in equilibrium with the zircon-quartz association at different temperatures and P=1 kbar. As seen from the diagram, the equilibrium concentrations of zirconium in the fluid with the constant HClo concentration decrease with temperature. All the three isotherms have the same slope, i.e., the ratio (d log mZraq/d log mHCl) =1. One can assume that under these conditions Zr(OH)3Clo is the dominant complex for which an equilibrium is written as
ZrSiO4 + HClo + H2O = Zr(OH)3Clo + SiO2solid (15)
Table 4. Conditions and results of the zircon solubility study in the system ZrO2-SiO2-H2O-HCl (P=1 kbar)
| Sample name | T, oC | During | Initial solution | pH in diluted solution | Equilibrium values of -lgmHCl | Equilibrium values of -lgmZr aq | -lgmHClo | +γ |
aH+ | lgK |
Co-CoO-Ag-AgCl-H2O-HCl |
||||||||||
| Zr-33(1) | 400 | 30 | H2O | 1.91 | 0.33 | - | 0.472 | 0.5176 | 6.76E-02 | 1.505 |
| Zr-33(2) | 400 | 30 | 1.5 m HCl | 1.97 | 0.37 | - | 0.517 | 0.5240 | 6.42E-02 | |
| Zr-33(3) | 400 | 30 | 0.1 m ZrCl4 | - | - | 1.98 | - | - | - | |
| Zr-33(4) | 400 | 30 | H2O | - | - | 2.02 | - | - | - | |
| Zr-51(1) | 500 | 14 | H2O | 1.30 | 0.12 | - | 0.126 | 0.6717 | 6.48E-02 | 2.255 |
| Zr-51(2) | 500 | 14 | 1.5 m HCl | 1.38 | 0.11 | - | 0.116 | 0.6701 | 6.56E-02 | |
|
Zr-51(3) |
500 | 14 | H2O | - | - | 2.40 | - | - | - | |
| Zr-51(4) | 500 | 14 | 0.1 m ZrCl4 | - | - | 2.36 | - | - | - | |
| Zr-147(1) | 600 | 12 | H2O | 1.96 | -0.27 | - | -0.269 | 0.8327 | 4.76E-03 | 2.590 |
| Zr-147(2) | 600 | 12 | 2m HCl | 1.98 | -0/23 | - | -0.229 | 0.8363 | 4.54E-03 | |
| Zr-147(3) | 600 | 12 | H2O | - | - | 2.31 | - | - | - | |
| Zr-147(4) | 600 | 12 | 0.1 m ZrCl4 | - | - | 2.37 | - | - | - | |
Ni-NiO-Ag-AgCl-H2O-HCl |
||||||||||
| Zr-24(1) | 400 | 30 | H2O | 1.78 | 0.60 | - | 0.7785 | 0.5616 | 4.75E-02 | 1.495 |
| Zr-24(2) | 400 | 30 | 1 m HCl | 1.74 | 0.54 | - | 0.7097 | 0.5517 | 5.14E-02 | |
| Zr-24(3) | 400 | 30 | H2O | - | - | 2.26 | - | - | - | |
| Zr-24(4) | 400 | 30 | 0.1 m ZrCl4 | - | - | 2.22 | - | - | - | |
| Zr-50(1) | 500 | 14 | H2O | 1.68 | 0.38 | - | 0.3871 | 0.7100 | 4.80E-03 | 2.270 |
| Zr-50(2) | 500 | 14 | 1 m HCl | 1.67 | 0.37 | - | 0.3770 | 0.7086 | 4.85E-03 | |
| Zr-50(3) | 500 | 14 | H2O | - | - | 2.63 | - | - | - | |
| Zr-50(4) | 500 | 14 | 0.1 m ZrCl4 | - | - | 2.68 | - | - | - | |
| Zr-150(1) | 600 | 14 | H2O | 1.60 | 0.18 | - | 0.1802 | 0.8694 | 2.83E-04 | 2.555 |
| Zr-150(2) | 600 | 14 | 2m HCl | 1.61 | 0.16 | - | 0.1602 | 0.8679 | 2.89E-04 | |
| Zr-150(3) | 600 | 14 | H2O | - | - | 2.70 | - | - | - | |
| Zr-150(4) | 600 | 14 | 0.1 m ZrCl4 | - | - | 2.75 | - | - | - | |
Fe3O4- Fe2O3 -Ag-AgCl-H2O-HCl |
||||||||||
| Zr-143(1) | 400 | 30 | H2O | 2.80 | 1.38 | - | 1.2773 | 0.6949 | 1.59E-02 | 1.300 |
| Zr-143 (2) | 400 | 30 | 2 m HCl | 2.94 | 1.39 | - | 1.7403 | 0.6966 | 1.57E-02 | |
| Zr-143 (3) | 400 | 30 | H2O | - | - | 3.03 | - | - | - | |
| Zr-143 (4) | 400 | 30 | 0.1 m ZrCl4 | - | - | 3.-4 | - | - | - | |
| Zr-141 (1) | 500 | 18 | H2O | 2.57 | 1.13 | - | 1.1449 | 0.8017 | 2.01E-03 | 2.245 |
| Zr-141 (2) | 500 | 18 | 2 m HCl | 2.60 | 1.20 | - | 1.2160 | 0.8088 | 1.85E-03 | |
| Zr-141 (3) | 500 | 18 | H2O | - | - | 3.39 | - | - | - | |
| Zr-141 (4) | 500 | 18 | 0.1 m ZrCl4 | - | - | 3.46 | - | - | - | |
| Zr-140 (1) | 600 | 14 | H2O | 2.54 | 1.03 | - | 1.0305 | 0.9187 | 1.06E-04 | 2.570 |
| Zr-140 (2) | 600 | 14 | 1m HCl | 2.52 | 1.02 | - | 1.0205 | 0.9183 | 1.07E-04 | |
| Zr-140 (3) | 600 | 14 | H2O | - | - | 3.57 | - | - | - | |
| Zr-140 (4) | 600 | 14 | 0.1 m ZrCl4 | - | - | 3.62 | - | - | - | |
It has to be mentioned that at T=400oC in the range of low HCl concentrations ( in the presence of a hematite-magnetite buffer) the slope of the curve is not 45o. It can be assumed that under these conditions along with Zr(OH)3Clo there are other zirconium chloride complexes in the solution.
Comparison of the solubility data for zircon and baddeleyite at the same T-P-mHCl conditions has shown that the concentration of zirconium in the solution in equilibrium with a zircon-quartz association is by 1-1.5 order of magnitude higher against an equilibrium with baddeleyite. This is in contradiction with thermodynamic data since the activity of zirconium in dioxide has to be higher than in salt. To understand this phenomenon we carried out additional runs with baddeleyite-zircon and zircon-quartz associations at T=400-600oC, P=1 kbar in H2O-HCl solutions ( in the absence of an Ag-AgCl buffer) using the capsule technique. After the run the quenched solutions were
15
analysed for the concentration of H4SiO4. The results are listed in table 5. Fig.6 gives the data reflecting the equilibrium concentrations of zirconium in the solution with baddeleyite-zircon and zircon-quartz associations at T=600oC, P=1 kbar, and mHCl=1M. The data were obtained by different techniques. Three fields are distinguished on the diagram, corresponding to: I stability of baddeleyite; II stability of zircon; III stability of zircon-quartz association. The stability field of baddeleyite is separated from the field of zircon with the line corresponding to the equilibrium:
ZrO2 + H4SiO4o = ZrSiO4 + 2H2O (16)
The fields of zircon and zircon-quartz association are separated with the line, corresponding to the solubility of quartz. It has been found that a baddeleyite-zircon equilibrium is reached at log mH4SiO4o = (-2.41 ¸ -2.47), herewith log mZraq = -4.20¸ -4.40. According to the data of McKenzie and Helgeson (1984 )[22], the concentration of SiO2aq in equilibrium with quartz for the given T-P parameters is 1.35 log un. In this case in the system H2O-HCl (without Ag-AgCl buffer) the experimentally defined concentration of zirconium in the solution in equilibrium with zircon-quartz association is 5.32 log un., but it is 2.78 log un. in the presence of the Ag-AgCl buffer. Fig.6 gives, also, the theoretical values of zirconium concentration in the solution, calculated from the thermodynamic equilibrium data (15). The calculation procedure was as follows.
The thermodynamic data for zircon were corrected from equilibrium (16), using the experimental data on the concentration of silicon in the solution in equilibrium with zircon and baddeleyite.
Free Gibbs energies of a Zr(OH)3Clo particle were calculated from our experimental data on baddeleyite solubility according to equilibrium (4) (see above).
Concentrations of zirconium in the solution in equilibrium with zircon-quartz association were calculated in accordance with eq.(15). The thermodynamic data for minerals and particles, used in the calculations, are listed in tables 6 and 7.
Comparison of the calculated and experimental (without Ag-AgCl buffer) data on zirconium concentration in the solution shows a fair agreement. The difference is no greater than 0.1 log.un. But where the Ag-AgCl buffer technique is employed the value for the same association is log mZraq=- 2.78. The difference may be due to the formation of complicated heteropolynuclear complexes of zirconium with silicon in the presence of silver which acts as a catalyst and, probably, stabilizes these complexes. The analogous picture of formation of heteropolynuclear tin-silicon complexes was observed in solubility studies of SnO2 [27]. It has to be mentioned that when baddeleyite solubility was studied with the Ag-AgCl buffer technique, in the absence of quartz in the system, no increase of zirconium concentration in the solution was observed.
The values of free Gibbs energy for zircon and baddeleyite were reconciled from equilibrium (16) using our experimental data on the concentration of silicon in the solution in equilibrium with baddeleyite-zircon association and on the concentration of zirconium in the solution in equilibrium with zircon-quartz association. The thermodynamic data for baddeleyite were borrowed from the work by Kelley (1960) [14], and for a H4SiO4 particle from the work by Walther and Helgeson (1977) [38], listed in table 7. The diagram of fig.7 gives in the coordinates T-logmH4SiO4 the concentrations of equilibrium H4SiO4aq, calculated from the thermodynamic data of various authors and our experimental data for the temperature range of 400-600oC at P=1 kbar. As suggested by an analysis of the diagram, our experimental data on equilibrium concentrations of H4SiO4o agree best with the calculated values obtained using thermodynamics for zircon and baddeleyite by Schuiling (1976), Matveev et. al.(1983) [34, 21]. The differences are no greater than 0,15 log un., which corresponds to 0.5-0.6 kcal/mole for the free Gibbs energy of zircon.
The obtained experimental data combined with the thermodynamic properties of the complexes enable more rigorous estimations of forms of zirconium transport in natural waters. Based on the existing ideas of the parameters of hydrothermal mineral formation , one can admit that temperatures 400-600oC at which we conducted runs on zircon and baddeleyite solubility are allowable both for acidic and alkaline metasomatites. The pressure P=1 kbar corresponds to optimal pressure for these formations but there are, indeed, higher and lower pressures. As for the composition of the acted hydrothermal solution, it is somewhat different for acidic and alkaline metasomatites.
According to the data on gas-liquid inclusions, the temperature range of acidic metasomatites (involving such formations as greisens, represented by quartz and muscovite; secondary quartzites, represented by quartz and andalusite, quartz and pyrophillite; quartz-feldspathic metasomatites, represented by quartz, orthoclase and hematite) spans 300 - 550oC, and the concentration of chlorides in a fluid can reach 20-40 wt pct which corresponds to their molar fraction of 0.1 - 0.2 [24]. For alkaline metasomatites ( including such formations as albitites, represented by albite, chlorite, algirite, riebeckite and others) the composition of the acted solution is characterized by lower chloride concentration and by the presence of hydrocarbonate-chloride-sodium component [20]. A distinctive feature of the process of acidic metasomatoses is the regime of increased chlorinity of the acted solution. According to the experimental data of Poop and Frantz(1980) [29] the concentration of hydrochloric acid in the hydrothermal process at the stage of acidic metasomatosis cannot exceed 10-3 – 10-2 mole/ kg H2O even at very high chloride concentrations.
So, based on our experimental data we can conclude that a significant increase of solubility of baddeleyite and zircon (see above) is observed only in HCl solutions with the concentration mHCl 0,8 mole/kg H2O, and prior to this concentration the content of zirconium in the solution is approximately 10-5 mole/kg H2O. As seen from the analysis, such high concentrations of HCl are absolutely unreal for hydrothermal postmagmatic processes, therefore transport of zirconium in the form of hydroxochloride complexes of zirconium is quite improbable at the stage of acidic metasomatosis. This is also supported by retrograde solubility of zircon and baddeleyite in a fluid in the temperature range 400 - 600oC and by a weak influence of additional saline loading in the form of NaCl on an equilibrium concentration of zirconium in a solution.
16
Table 5. Conditions and results of experimental study on baddeleyite-zircon and zircon-quartz associations in hydrothermal solution at P=1 kbar
Initial solution |
Final solution |
|||||||||
Charge, mg |
T, oC | During t, days | mHCl mole/ kgH2O |
-lgmHClo | -lgmSiO2 aq | -lgmZr aq | lgK | Phase composi- |
||
| ZrO2 | ZrSiO4 |
SiO2 | ||||||||
| 16.4 | - | 6.0 | 600 | 12 | 1mHCl | 0.119 | - | 4.783 | Zrc+Bd Zrc+Bd+Q Zrc+Bd+Q Zrc+Bd+Q Zrc+Bd+Q Zrc+Bd+Q Zrc+Bd+Q Zrc+Bd Zrc+Bd Zrc+Bd |
|
| 16.4 | - | 8.0 | 600 | 12 | 1mHCl | 0.119 | - | 4.741 | ||
| 16.4 | - | 8.5 | 600 | 12 | 1mHCl | 0.119 | 1.962 | 3.857 | ||
| 16.4 | - | 9.0 | 600 | 12 | 1mHCl | 0.119 | 1.660 | 3.898 | ||
| 16.4 | - | 9.5 | 600 | 12 | 1mHCl | 0.119 | 1.484 | 4.302 | ||
| 16.4 | - | 10.0 | 600 | 12 | 1mHCl | 0.119 | 1.359 | 4.662 | ||
| 16.4 | - | 14.0 | 600 | 12 | 1mHCl | 0.119 | - | 4.862 | ||
| 20.0 | 20.0 | - | 600 | 10 | 0.01mHCl | 2.07 | 2.468 | 4.608 | 2.47 | |
| 20.0 | 20.0 | - | 600 | 10 | 0.01mHCl | 2.07 | 2.414 | 4.835 | 2.41 | |
| 20.0 | 20.0 | - | 600 | 10 | 1mHCl | 0.119 | - | 4.586 | ||
| - | 20.0 | 20.0 | 600 | 10 | 1mHCl | 0.119 | - | 5.32 | -5.20 | Zrc+Q Zrc+Bd Zrc+Bd Zrc+Bd Zrc+Bd |
| 20.0 | 20.0 | - | 400 | 24 | 0.01mHCl | 2.59 | 3.272 | - | 3.27 | |
| 20.0 | 20.0 | - | 400 | 24 | 0.01mHCl | 2.59 | 3.324 | - |
3.32 |
|
| 20.0 | 20.0 | - | 500 | 12 | 0.01mHCl | 2.11 | 2.760 | - | 2.76 | |
| 20.0 | 20.0 | - | 500 | 12 | 0.01mHCl | 2.11 | 2.703 | - | 2.70 | |
Table 6. Available thermodynamic data for ZrO2 and ZrSiO4
-ΔHo298 kcal/mol |
-ΔGo298 kcal/mole |
So298 cal/(mole Grad) |
Co298 cal/(mol. Grad) |
a |
b |
c |
T, interval (K) |
Refe- |
| ZrO2 | ||||||||
| - | - | - | 13.39 | 16.64 | 1.80 | -3.36 | 298-1478 | [4] |
| 263.04 | 249.27 | 12.12 | 13.40 | 16.64 | 1.80 | -3.36 | 298-1478 | [23] |
| 262.978 | - | 12.04 | 13.37 | 68.329 | 9.081 | -13.438 | 298-1445 | [32] |
| 262.3 | 248.505 | 12.04 | 13.41 | 90.70 | 4.388 | -8.133 | 298-1478 | [27] |
ZrSiO4
| - | 459.02 | - | - | - | - | - | - | [31] |
| - | - | - | 23.55 | 31.48 | 3.92 | 8.08 | 298-1800 | [4] |
| 488.050 | 460.325 | - | - | - | - | - | 1300-1400 | [20] |
| 485.994 | 458.626 | 20.08 | 13.41 | - | - | - | - | [27] |
| 484.0 | 456.684 | 20.2 | 12.56 | 31.48 | 3.92 | 8.08 | 298-1800 | [14] |
| 475.7 | 448.4 | 20.2 | 12.56 | 31.48 | 3.92 | 8.08 | 298-1800 | [23] |
| 486.138 | 458.891 | 20.086 | - | - | - | - | - | [32] |
Table 7. Free Gibbs energies of formation of minerals and particles in a solution(P=1kbar).
Mineral |
Formula |
mol.volume cal/bar |
-ΔGfo kcal/mol |
Reference |
||
| 400oC | 500oC | 600oC | ||||
| Bd | ZrO2 | 0.5055 | 232.177 | 227.699 | 223.251 | [14] |
| Zrc | ZrSiO4 | 0.9383 | 426.944 | 417.709 | 408.886 | Authors data |
| Q | SiO2 | 0.5423 | 188.334 | 184.047 | 179.808 | [35] |
| Zr(OH)4o | 304.407 | 288.429 | 276.856 | Authors data |
||
| Zr(OH)3Clo | 283.560 | 271.431 | 259.492 | Authors data |
||
| Zr(OH)2Cl2o | 259.560 | 248.113 | 237.316 | Authors data |
||
| Zr(OH)Cl3o | 235.548 | 225.042 | 214.620 | Authors data |
||
| ZrCl4o | 211.291 | 201.618 | 191.605 | Authors data |
||
| H4SiO4o | 268.653 | 257.782 | 247.075 | [35] |
||
| HClo | 18.719 | 17.661 | 15.727 | [7] |
||
| H2O | 42.807 | 39.571 | 36.435 | [5] |
||
Conclusions. The principal results obtained on solubility of baddeleyite in a HCl fluid under high T-P conditions are the following:
Solubility of baddeleyite in pure water and diluted solutions of hydrochloric acid is insignificant and is, practically, independent on the concentration of HCl to 0,2 m. The dominant complex in this concentration region of HCl is Zr(OH)4o. In the concentration region of HCl higher than 0,2 m the solubility of baddeleyite increases drastically and the dominant complexes are mixed hydroxochloride complexes of zirconium of the type Zr(OH)4-nClno, where 'n' varies from 0 to 4. The dominant complex in the concentrated region (mHCl 0.8 m) is ZrCl4o.
The solubility of baddeleyite at a constant concentration of HCl decreases with temperature. The effect of pressure on the solubility of baddeleyite is insignificant.
The principal results obtained on the solubility of zircon in the presence of quartz are the following:
The concentration of zirconium in a solution in equilibrium with a zircon-quartz association in the presence of a Ag-AgCl buffer is higher than in equilibrium with baddeleyite under the same T-P and mHCl conditions. This is due to the formation of heteropolynuclear complexes of zirconium with silicon in the presence of silver.
17
The concentration of zirconium in a solution in equilibrium with a zircon-quartz association decreases with temperature.
The values of free Gibbs energies are refined for zircon by using the experimental data on the concentration of H4SiO4o in a solution in equilibrium with a zircon-quartz association.
The transport of zirconium and its deposition at the hydrothermal mineral formation stage occurs in solutions of increased alkalinihy with the participation of hydrocarbonate chloride-sodium solutions. The occurrence of sodium ions, hydrocarbonate anions in the acted solution at the stage of alkaline metasomatosis is responsible for clear rare-metal mineralization.
References:
18