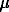 cm in size. The strontium-loaded biomass was separated from water by microflotation. The following reagents were used as coagulating agents: (1)
cm in size. The strontium-loaded biomass was separated from water by microflotation. The following reagents were used as coagulating agents: (1)Radioactive wastes
#Suvorova V.A. ,* Pertsov N.V. ,** Akhmedzhanova G.M. ,* and Kotelnikov A.R. * Ceramic matrices synthesized from flotation slimes with high content of strontium.
key words[cell flotation immobilization radioactive strontium ceramic disposal]
Institute of Experimental Mineralogy, Russian Academy of Sciences, Chernogolovka, Moscow Region, Russia ;** Institute of Biocolloidal Chemistry, National Academy of Sciences of the Ukraine, Ukraine
The purpose of the work is the synthesis of matrix material for binding strontium in raw flotation slime, which is the product of the biocolloidal technology of extraction of radionuclides from waste water. Based on studying the leaching of strontium, we evaluated a possibility of using solid model compositions obtained by ceramization of mixtures of Sr salts and kaolinites for long time immobilization of 90Sr under conditions of controlled storage.
Liquid radioactive waste (RW) of the nuclear fuel cycle or formed under emergency conditions is highly dangerous for storage. Scientists from the Ukraine and Russia have developed a method for purification from radionuclides of contaminated water accumulated under the emergency reactor of the fourth block of the Chernobyl nuclear power station. The method is based on the treatment of water by specially selected metallophilic microorganisms followed by separation of the dispersed phase (concentrator of radionuclides) by flotation.
A decrease in the potential danger of RW and their volume can be achieved by compacting, i.e., solidification. We attempted to obtain a solid alumosilicate matrix from Sr-containing flotation slimes, products of the microorganism flotation, by their ceramization. For this purpose, a sample of the raw flotation slime containing about 10 wt.% Sr was used as the material for studies. The sample was kindly presented by our colleagues from the Institute of Biocolloidal Chemistry of the National Academy of Sciences of the Ukraine, where it has been obtained by mixing of the active mud (microorganisms) with an aqueous solution of strontium chloride (200 mg/l) for 1-2 h. Strontium was bound by living cells combined in floccules 50-100 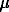 cm in size. The strontium-loaded biomass was separated from water by microflotation. The following reagents were used as coagulating agents: (1)
cm in size. The strontium-loaded biomass was separated from water by microflotation. The following reagents were used as coagulating agents: (1)
# This work was supported by the RFBR grant N 95-05-65886
95
aluminum sulfate Al2(SO4)3*10H2O (90.66 g/l); (2) a solution of NaOH for alkalization to pH = 8.5; and (3) cationic surfactant ethonium, 1,2-[N,N-bisdimethyl-N,N'-bisdecylacetate ethylenediammonium] dichloride (8 mg/l).
The starting flotation slime (50 ml) was dried at 110oC to the solid residue, which was burnt at 700oC. The weight of the dry residue was 226 mg, and the microprobe analysis of the powder showed that it retained a large amount of Cl (21.03%) along with silicon oxides (4.68%), aluminum (7.43%), calcium (2.39%), phosphorus (10.60%) and a high content of SrO (48.12%).
The latter means that strontium is retained in the form of chloride and, hence, cannot be introduced to the alumosilicate matrix, which is the most stable in retaining alkali-earth elements. For ceramization, strontium should be in the form of compounds that easily decompose at high temperatures: carbonates or nitrates. The treatment of the slime with nitric acid gave the product containing no chlorine, but a small amount of the slime could not provide further experiments, and we performed model experiments on introduction of strontium to kaolinites.
Strontium was introduced by ceramization of calcinate from SrCO3 or Sr(NO3)2 and natural kaolinites with addition of water (1 wt.%) in sealed platinum ampules at 500, 700, and 1100oC and 1000 atm. The starting charge was prepared by prolonged stirring followed by drying at 110oC of a mixture of kaolinite (stirred under alcohol) and salt. In all cases, the starting charge placed in an open crucible was calcined at 700oC for 1-3 days. The conditions and results of the experiments are presented in Table 1.
Table 1. Conditions and results of experiments on ceramization
|
Entries nos |
Starting charge |
Ceramization parameters |
Formulas of compounds (products of experiments) by microprobe analysis data |
||
|
ToC |
P/atm |
days |
|||
|
S-4 |
SrCO3 + kaolinite |
500 |
1000 |
3 |
ceramics was not obtained |
|
S-5 |
SrCO3 + kaolinite |
700 |
1000 |
3 |
(Na0.007K0.055Sr0.297)0.357(Al2.129Si2.239)4.368O24 |
|
S-6 |
SrCO3 + kaolinite |
1100 |
1000 |
3 |
(Na0.008K0.058Sr0.902)1.068(Al2.182Si2.114)4.297O24 |
|
S-7 |
SrCO3 + kaol. wool |
1100 |
1000 |
3 |
(Na0.038K0.009Sr0.787)0.834(Al2.060Si2.037)4.197O24 |
|
S-8 |
Sr(NO3)2 + kaol. wool |
1100 |
1000 |
3 |
(Na0.023K0.007Sr1.015)1.015(Al2.060Si1.982)4.042O24 |
Table 2. Rates of strontium leaching logV (g/m2*day) from samples obtained by ceramization of mixtures of Sr salts with kaolinites
|
Samples nos |
Initial content of Sr, wt.%; |
d, g/cm3 |
Por, %; |
Duration of leaching, days |
|||
|
0-1 |
1-7 |
7-14 |
14-18 |
||||
|
S-5 |
14.23 |
2.7 |
12 |
1.0 |
-0.03 |
- |
- |
|
S-6 |
21.75 |
2.3 |
23 |
0.24 |
-0.65 |
- |
- |
|
S-7 |
20.36 |
2.4 |
20 |
0.76 |
-0.06 |
- |
- |
|
S-8 |
26.84 |
2.7 |
20 |
0.79 |
-0.04 |
-0.76 |
-0.54 |
After quenching, the products were compact small cylinders 30-40 mm long with the diameter of 3-5 mm. Reflections of strontium feldspar were observed in X-ray patterns of these products. The ceramic samples obtained in these experiments had the density of 2.4-2.7 g/cm3 (85-92% of the theoretically calculated value for strontium feldspar) and percentage of open porosity of 12-20. The quality of the ceramics was evaluated from leachability of elements from the samples in distilled water at 90oC (MAGATE MCC-1 test). The results of the tests are presented in Table 2.
It is seen from the results presented that the charge of Sr(NO3)2 and kaolinite wool gives a better ceramics. Only 0.86% strontium is transferred to the leaching medium during experiments. The average value of the leaching rate achieved after 22 days is 0.2 g/m2*day. The considerable rates of sodium leaching on the 40th day of 2.4 g/m2*day indicate to a weak stability of the matrix material as a whole.
After 40 days of exposure, the data on leaching obtained for Sr-containing charge-based ceramics, whose stoichiometry corresponds to strontium anorthite, showed the average rate of strontium leaching of 0.2 (logV = -0.5) g/m2*day, which is twofold better than that from borosilicate glasses [1], the more so the initial content of strontium in our samples is considerably higher. Despite the high porosity (~20%), which determines fast leaching of strontium, the sample retains a low percentage of Sr leaching, which makes our method promising for solidifi-
96
cation of flotation slimes that purify aqueous pools to forms appropriate for disposal in rocks.
References:
# Koval'skii A.M.,* Kotelnikov A.R.,** Akhmedzhanova G.M.,** Suvorova V.A.,** and Tikhomirova V.I.** (Na,Sr)-feldspars as potential matrix material for immobilization of strontium radionuclides.
key words [Na,Sr-feldspar matrix strontium radionuclide]* Department of Geology, Moscow State University, Moscow, Russia; ** Institute of Experimental Mineralogy, Russian Academy of Sciences, Chernogolovka, Moscow Region, Russia
It has been previously shown (Akhmedzhanova et al., 1993; Kotelnikov et al., 1995) that natural feldspars can be compared to Synroc samples by stability to leaching processes (Ringwood et al., 1988) and can serve as potential matrix materials for radionuclide fixation. Well-developed isomorphic substitutions of more than 20 elements and their stability in the Earth crust rocks are evidence in favor of their use as mineral-concentrators of radionuclides. Kotelnikov and coworkers (1995) have developed a method for synthesis of (Na,Sr)-feldspars from NaX zeolites preliminarily saturated with strontium. However, the necessity to use high PT-parameters (1000oC, P = 1-2 kbar) for synthesis of feldspars restricted the possibility of using this material as a matrix. Therefore, the process of the phase zeolite --> feldspar transformation should be studied in more detail in order to decrease the PT-parameters of this phase transition. Information on the thermodynamic properties of (Na,Sr)-feldspars is required to evaluate the behavior of solid solutions in the matrix-solution-host rock system.
Experimental study of the solid solutions of (Na,Sr)-feldspars
1. The study of the phase transformation Sr-substituted NaX zeolite  feldspar
feldspar
The Sr-substituted zeolite, whose composition corresponded to the formula (calculated per 8 oxygen atoms) Na1.58Al1.89Si2.13O8, was used as the starting material. Points of phase transitions (dehydration of zeolite, transformation zeolite  feldspar) were determined by the DTA method. The phase transformation process is complicated: at first, zeolite is dehydrated at t ~ 230-580oC, then at t ~ 920oC the zeolite
feldspar) were determined by the DTA method. The phase transformation process is complicated: at first, zeolite is dehydrated at t ~ 230-580oC, then at t ~ 920oC the zeolite  feldspar transition occurs. Thus, the transformation of zeolite into feldspar occurs at the atmospheric pressure at temperature about 900oC.
feldspar transition occurs. Thus, the transformation of zeolite into feldspar occurs at the atmospheric pressure at temperature about 900oC.
2. The study of the sodium and strontium distribution between feldspar and fluid
Fig.1. Distribution of Na,Sr between (Na,Sr)-feldspar and water-salt fluid. The data: 1,2 - of this work (1- starting compositions of feldspar and fluid before the experiment; 2 - equilibrium compositions of feldspar and fluid after the experiment 700oC, 2 kb); Lagashe and Dujon (1987); 3 - 600oC, 1.5 kb, 4 - 750oC, 2 kb; 5-Kotelnikova at all (1987) 800oC, 2 kb; 6 - cross sizes determing compositions of coexisting phases.
Experiments on the Na and Sr distribution between feldspar and fluid were carried out by the ampule method on hydrothermal high-pressure apparatuses with external heating and the cool seal at 700oC and Pfl = 2 kbar. The accuracies of the temperature and pressure control were +5oC and +50 bar, respectively. The duration of experiments was up to 30 days, according to the data of Lagache and Dujon (1987), this duration is sufficient for achievement of the equilibrium. After experiments, solid phases were analyzed on a microprobe, and solutions were analyzed by the AAS method. The X-ray phase analysis revealed only feldspar in the products, which indicates the following reaction:
2NaAlSi3O8 + SrCl2 = SrAl2Si2O8 + 2NaCl + 4SiO2 (1)
The experimental results are shown in Fig. 1. It is seen that the strontium distribution between feldspar and fluid is not ideal. Strontium enriches feldspar as compared to the fluid in the whole range of compositions. Based on the experimental data, we calculated the dependence of the logarithm of the strontium distribution coefficient on the feldspar composition: according to our data (700oC and 2 kbar) and results of Lagache and Dujon (1987) for 750oC and 2 kbar, the lnKD function can be presented by two straight sections: below XSrFsp ~~ 0.7 the lnKD are constant and equal to 3.2 + 0.5, and at XSrFsp > 0.7, the concentration dependence of the distribution coefficients gains the following form:
# This work was financially supported by the Russian Foundation for Basic Research (Project No. 97-05-65886).
97
lnKD = 0.32 + 4.3 * XSrFsp (2)
3. Refinement of unit cell parameters
Unit cell parameters were refined on the basis of X-ray studying the solid solutions of (Na,Sr)-feldspars synthesized. The X-ray recording was carried out on DRON-UM1 and ADP-2 diffractometers (CoKa and CuKa radiation Fe and Ni filters, respectively) within the angle range from 10 to 100 deg2 with the scan velocity of 0.8 deg 2/min. Metallic silicon was used as the internal standard. Unit cell parameters were calculated by the LCC (Burnham, 1991) and KRIST (developed by SIC "Burevestnik," St. Petersburg) programs. Unit cell parameters were refined by 24-35 reflections. The results of determination of the unit cell parameters (concentration dependences) are shown in Fig. 2 (a-e: [a, b, c,  , V]). The studies performed made it possible to refine substantially the data of Bambauer et al. (1984).
, V]). The studies performed made it possible to refine substantially the data of Bambauer et al. (1984).
4. Calculation of excessive mixing functions
The parameters of the Margulus model for calculation of excessive energies of mixing of binary solid solutions of (Na,Sr)-feldspars were determined for 700oC: W1 = 2.54 (50); W2 = 0.65 (11) kJ/mol. The parameters of the Margulus model for calculation of the mixing volume are the following: W1 = 3.29(4) and W2 = 5.15(5) cm3/mol.
5. Synthesis of (Na,Sr)-feldspars by methods of sorption and phase transformations
Feldspars were synthesized by the following scheme: Precipitation of Sr on zeolite (sorption at 25oC)  dehydration of zeolite at 580oC
dehydration of zeolite at 580oC  phase transformation of zeolite into feldspar (annealing at ~900oC).
phase transformation of zeolite into feldspar (annealing at ~900oC).
Pellets were molded from Sr-substituted NaX zeolite, heated in a resistance furnace to 900oC, and exposed at this temperature for 2 days. As a result, ceramic samples with the density of 2.71 g/cm3 (93% of the theoretical density) were obtained. The results of X-ray and microprobe analyses showed that the unit cell parameters of the feldspar synthesized corresponded to those of the solid albite-strontium celsian solution, and its composition corresponded to the formula Na0.29Sr0.71Al1.72Si2.29O8. Thus, the composition of the (Na,Sr)-feldspar synthesized is identical to that of the strontium-substituted NaX zeolite. The rates of Sr leaching for the samples obtained were determined from the procedure of the MCC MAGATE test.
1. The experimental results are shown in Fig. 3. It is seen that the rate of strontium leaching from (Na,Sr)-feldspars is comparable to that of the Sr leaching from the Synroc-C matrix (most stable of all matrix materials presently developed) and by two orders of magnitude lower than the rates of leaching of alkali and alkali-earth elements from borosilicate glasses.
Fig.2. Dependence of the parameters of unit cell of (Na,Sr)-feldspars on the compositions of the solid solutions. The data: 1 - Kroll et all. (1986); 2 - Schops (1980); 3-Nager (1974); 4-this work; 5-Bambauer et al. (1984).
Fig.3. Kinetic curves of the logarithms of the rates of strontium leaching from different matrix materials.
98
2. The special experiments showed that the processes of leaching elements from feldspars is incongruent: the rate of the silica leaching exceeds leaching rates of other elements, i.e., feldspars are desilicated. It was experimentally determined that the concentration of strontium in the hydrothermal solution equilibrium with (Na,Sr)-feldspars (at 250oC and pressure of saturated vapor) decreased when quartz was added to the reaction volume. Therefore, we developed special double-layered matrix materials, which are a pseudo-granite rock based on strontium-containing feldspars and quartz surrounded with a quartz shell. This double-layered matrix prevents incongruent leaching of feldspar, and the quartz shell serves as an additional barrier that decreases the medium action on the matrix and decreases the migration of elements from the matrix. At the same time, the matrix satisfies the principle of phase and chemical correspondence in the matrix-host rock system (Kotelnikov et al., 1994), since quartz and feldspar are the main rock-forming minerals of many types of rocks.
References:
Dunaeva A.N. Calculation of activity of solid solution components in modeling of sorption and coprecipitation of heavy metals and radionuclides in natural systems.
key words [thermodynamic calculation]V. I. Vernadskii Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences, Moscow, 117975 Russia
An apparatus of the thermodynamics of solid solutions, which models the distribution of microcomponents between an aqueous solution and a mineral-sorbent, is used for simulation of sorption and coprecipitation processes in complex polymineral natural media.
The standard chemical potential and activity coefficient of the sorbed component of the solid phase were calculated from the available experimental data on sorption of heavy metals and radionuclides in simple systems. The standard chemical potential was determined from the reaction constant calculated in the region of low concentrations. The Margulus model for regular and subregular solid solutions is most often used to calculate the activity coefficients of solid phase components. However, due to the specificity of the available initial experimental data (as a rule, small number of distribution points obtained in a very narrow concentration range, different accuracies and reliability of data), the calculation of the Margulus parameters by the nonlinear least-squares selection method often gives values of the energy parameters, which are characterized by great errors and are not satisfactory in the physical sense. Therefore, in some cases, a ratio of the activity of the component to the solid phase composition was calculated without the solid solution model.
For example, for the reaction of the type
A(s.ph.) + B(sol) = B(s.ph.) + A(sol),
the equilibrium constant can be written in the form
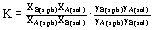 Here X and
Here X and 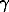 correspond to the molar fractions and activ-
correspond to the molar fractions and activ-
99
ity coefficients of the components of the system. In the case of isovalence ion exchange and at low ion strengths of an aqueous solution, A(sol)/B(sol) = 1. Then, determining the distribution coefficient of components A and B between the solid phase and the solution as
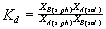
and going to the logarithmic form, we obtain
lnK = lnKd + lnXB(s.ph.) - lnXA(s.ph.) and
dlnKd + dlnXB(s.ph.) - dlnXA(s.ph.) = 0
It follows from the Gibbs-Duhem equation that
XA(s.ph.)*dln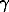 A(s.ph.) + XB(s.ph.)*dln
A(s.ph.) + XB(s.ph.)*dln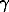 B(s.ph.) = 0.
B(s.ph.) = 0.
Combining this expression with the previous one, we obtain
ln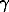 A(s.ph.) = ò
XB(s.ph.)dlnKd
A(s.ph.) = ò
XB(s.ph.)dlnKd
ln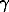 B(s.ph.) = ò
-XA(s.ph.)dlnKd.
B(s.ph.) = ò
-XA(s.ph.)dlnKd.
Thus, the problem on calculation of the activity coefficients of the components in the solid solution is reduced to the analytical description of the dependence of the distribution coefficient on the composition of the coexisting phases and integrating the polynomials obtained. This method for the calculation of the activity coefficients of the solid solution components was applied previously by different authors to different magmatic mineral associates and gave satisfactory results for simultation of sorption processes in the systems natural waters--minerals of soils and grounds.
The experimental sorption isotherms compared to the simulation results are presented in Figs. 1 and 2. The experiments performed (Comans et al., 1991, 1987) reflect the processes of sorption of Cs and Cd on potassium- and calcium-saturated illites. Both plots demonstrate good agreement between the experimental and calculated data.
Fig.1. Sorption of cesium on K-saturated illite. 1-absorption data; 2 - description data; 3-solid line is calculated from computer modelling
Fig.2. Sorption of cadmium on Ca-illite. 1-experimental data; 2-data obtained from computer modelling
100