VII. Crystall growth, stability and properties of minerals
Balitsky V.S. Preparation of new materials by synthesis and transformation of minerals.
1. A laboratorial technique was developed for the preparation of P-bearing quartz crystals tending to turn pink under ionizing irradiation. Pink quartz crystals are very rare in nature and are of great jewelry value (comparable with amethyst, topaz, and tourmaline in cost). The principally different origins of the pink color of quartz were experimentally established. The color of the high-temperature (>500oC) quartz from pegmatites is due to Ti3+ incorporated in quartz structure, while the color of the low-temperature (< 320 C) hydrothermal quartz results from the substitution of Si4+ in defective tetrahedra for PO43+. Thus, the synthetic pink quartz can be industrially pro-
44
duced by applying the traditional technology of hydrothermal crystal growth.
2. A technique for the reproducible and controlled growth of high-temperature 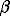 -quartz on a seed was developed. The results obtained enable the systematic experimental study of the properties and physicochemical conditions of
-quartz on a seed was developed. The results obtained enable the systematic experimental study of the properties and physicochemical conditions of 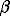 -quartz origin in nature.
-quartz origin in nature.
It was experimentally discovered that under direct temperature gradient, silica transfer and quartz crystal growth from supercritical aqueous fluids can occur in both high-density (r > 0.33 g/cm3) and low-density (r = 0.05-0.15 g/cm3) solutions. At a given temperature, the direction of silica transfer during the crystal growth, which is responsible for the location of dissolution and precipitation zones in the thermogradient field, is dictated by density, composition, and acidity of the fluid. This fact should be taken into account in paleoreconstructions of natural mineral formation and development of new methods for the growth of high-temperature quartz crystals.
We examined the growth rates of different faces of 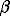 -quartz crystals grown on seeds from supercritical aqueous fluids at temperatures to 900oC and pressures to 5 kbar. The {1010} hexagonal prism and {1011}, {2021}, {3031}, and other {h0hl} hexagonal bipyramids were established to be stable crystal forms. Crystal habit varies from short prismatic (high-density fluids) to elongated bipyramidal (low-density fluids), depending on the relation between the growth rates of the faces. The ordinary bipyramidal habit formed only by {1011} faces, typical of
-quartz crystals grown on seeds from supercritical aqueous fluids at temperatures to 900oC and pressures to 5 kbar. The {1010} hexagonal prism and {1011}, {2021}, {3031}, and other {h0hl} hexagonal bipyramids were established to be stable crystal forms. Crystal habit varies from short prismatic (high-density fluids) to elongated bipyramidal (low-density fluids), depending on the relation between the growth rates of the faces. The ordinary bipyramidal habit formed only by {1011} faces, typical of 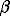 -quartz crystals grown from silicate melts, was not observed.
-quartz crystals grown from silicate melts, was not observed.
3. An original technology was developed for the growth of dichromatic amethyst--citrine quartz (ametrine), which is regarded to be one of the most valuable and rare colored quartz varieties. The crystallization parameters of ametrine were evaluated from the thermic stability of the amethyst color centers and morphological stability of the growing faces. The crystallographic directions of growth were determined with consideration for the coexistence of two types of crystal faces that entrap selectively structural and nonstrucural Fe impurities. Seed shape and size were selected so that amethyst and citrine growth sectors took approximately equal volumes in growing ametrine crystals, and the crystals obtained were best suitable for various applications. This is the pioneering technology for the growth of bicolored amethyst-citrine single crystals, which are very rare in nature and are only found at the Anaha Deposit (Bolivia).
4. Chemical interaction and thermodiffusion in sapphires were studied at 300-1700oC and 1 atm and 1300oC and 5-7 kbar in inert gas (argon) atmosphere. The step mechanism was established for the thermodiffusion coloring of colorless corundum in contact with cobalt and iron. At high temperatures (1100-1700oC), corundum single crystals exposed to air are covered by Co- and Fe-spinel films, which subsequently dissolve in the crystal to yield the spinel solid solution in corundum.
The thermodiffusion technique of blue and green coloring of natural and synthetic corundum crystals was successfully tested in the Physical Energy Institute (Obninsk) and now can be used as an industrial technology.
5. The effect of isovalent impurities on the 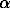 -
-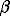 transition point of (Si(1-x)Gex)O2 solid solutions with quartz structure was studied. The transition point was shown to range from 571oC for pure quartz to 780oC for quartz with 21 wt % GeO2. The thermodynamics of the phase transition in the solid solutions were first determined by scanning calorimetry.
transition point of (Si(1-x)Gex)O2 solid solutions with quartz structure was studied. The transition point was shown to range from 571oC for pure quartz to 780oC for quartz with 21 wt % GeO2. The thermodynamics of the phase transition in the solid solutions were first determined by scanning calorimetry.  H(
H(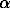 -
-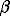 ) ranges from 0.45 kJ/mol for pure quartz to 0.12 kJ/mol for quartz with 6.2 wt % GeO2; S(
) ranges from 0.45 kJ/mol for pure quartz to 0.12 kJ/mol for quartz with 6.2 wt % GeO2; S(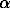 -
-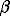 ) ranges from 0.53 J/mol K to 0.03 J/mol K, respectively.
) ranges from 0.53 J/mol K to 0.03 J/mol K, respectively.
The electron structure of (Si(1-x)Gex)O2 solid solutions was studied by X-ray fluorescence spectroscopy. The absence of significant electron interaction between the quartz matrix (SiO2)n and GeO2 impurity confirms the molecular type of bond and the block structure of GeO2-SiO2 solid solutions.
Dislocations, defects of different types, and Brazil twins were detected in single crystals of the Ge-bearing quartz solid solution by the X-ray topography analysis with synchrotron irradiation (Institute of Nuclear Physics, Siberian Division, Russian Academy of Sciences). Data obtained enable the quality control over the crystals and the optimization of the synthesis parameters (S.L. Sorokina and V.S. Balitsky).
6. Phase formation in the Ga2O3--GeO2 system was experimentally studied in the temperature range 400-1300oC and pressures up to 8 kbar for the simulation of phase transformations of andalusite (Al2O3--SiO2 system). It was shown that under the solid-phase synthesis conditions, phases with andalusite (1 atm, 1000-1300oC), disthene (5 kbar, 600oC), and sillimanite (8 kbar, 800oC) structures form in the Ga2O3--GeO2 system. Solid solutions with wollastonite structure were synthesized in the SiO2--GeO2--CaO system at 1000-1300oC (S.L. Sorokina and V.S. Balitsky).
References:
- Balitsky V.S. (1991) Colored stones: research on treatments and synthetics, Proc. Intern. Gemol. Symp. GIA, Santa Monika, pp. 125-127.
- Balitsky V.S. (1991) Gemstone occurrences in the USSR, Proc. Intern. Gemol. Symp. GIA, Santa Monika, pp. 65-66.
- Balitsky V.S. (1991) Structural-morphological and spectrooptical characteristics of synthetic iron-bearing quartz, Proc. Intern. Gemol. Symp. GIA, Santa Monika, pp. 153-155.
- Balitsky V.S. and Bublikova T.M. (1991) Present and future of synthetic malachite, Proc. Intern. Gemol. Symp. GIA, Santa Monika, pp. 151-152.
- Balitsky V.S. (1994) Synthesis and modification of minerals. // Experimental Problems in Geology, Moscow: Nauka, , p. 624.
- Balitsky V.S., Sorokina S.L., Chichagov A.V., and Bondarenko G.V. (1994) Growth of high-temperature quartz from hydrothermal solutions.// Experimental problems in geology, Moscow: Nauka, , pp. 625-627.
- Balitsky V.S. and Zhizheiko I.A. A method for corundum coloring, Patent N.2036984, June 9, 1995, Russian Federation, Priority since March 14, 1991.
45
- Balitsky V.S. and Sorokina S.L. A method for the synthesis of quartz crystals, Inventor's certificate no. 1736199, January 22, 1992.
Novikov G.V., Sipavina L.V. Germanium analogs of mantle silicate phases: structures and phase transitions.
The features of the structures and chemical bonds Fe-O in the Ca-Fe-Mg germanates with the pyroxene, olivine, spinel and garnet structures have been studied by the X-ray powder diffraction and nuclear gamma-resonance (Mössbauer effect) methods. The close affinity between the geometries of coordination polyhedra in these germanates and silicates was supported, and attested germanates as a good model system of "geometrically determined " silicates with a rigid tetrahedral group TO4. In particular, a close similarity of iron ions electron structure was determined in the Fe-Mg silicates and germanates with the olivine structure. The same similarity was found in the chain Ca-Fe and Fe-Mg silicates and germanates with the pyroxene structure. The temperature dependencies of local fields in germanates with olivine and spinel structures indicate the stability of electron structure of iron ions in the wide range of composition and temperatures and their close affinity in germanates and silicates. At the same time the olivine-spinel structural transition provoked in germanates by the Mg-Fe substitution is accompanied by an essential change in the electron structure of iron cations.
In germanates of the (Fe1-xCax)GeO3 join with C2/c pyroxene structure, it was first found the phase transition without changing space group which was accompanied by great volume effect and alteration of coordination number of cation in M2 position. At the same time, it was shown by the nuclear gamma-resonance method that at this phase transition just the electronic structure of iron ions in M1 position shows a notable change. These two structurally distinct phases of Ca-Fe germanates are structural analogs of unstable ferrosilite phases with the high-pressure and high-temperature structure modifications.
The results obtained show an important role of the M1 octahedra in the structural transformation in Ge-pyroxenes and explain, in particular, strong effect of cation substitution in the M1 position on the structural properties of pyroxene structures in general. In the corresponding silicate phases, this phenomenon can not be fixed by the similar direct experiments.
The magnetic structure of FeGeO3 was first determined in the temperature range 1.7-300 K. Magnetic nature of the low-temperature phase transition in this Ge-analog of ferrosilite was proved. It was shown by the neutron diffraction method that at 1.7-12 K magnetic moments of iron ions were oriented along the crystallographic c axis and at 14-53 K - along the b axis coinciding with gradient of the local electric field.
The results obtained on the local fields in germanates and silicates allow to essentially extend the database for the analysis of mechanisms and physical motives of phase transformations in mantle related minerals. Moreover, the germanates have often the structures with one magnetic sublattice which is a model for understanding magnetic properties of pyroxenes.
References:
- Novikov G.V., Hafner S.S., Sipavina L.V., Shibaev A.P. (1994) Hyperfine interactions and magnetic transition in monoclinic FeGeO3. // Experiment in Geosciences, V.3, N.1, pp.10-20.
- Novikov G.V., Sipavina L.V., Shibaev A.P. Local fields on 57Fe in triclinic FeSiO3. (1994) // Experiment in Geosciences,. V.3, N.2, pp.1-8.
- Novikov G.V., Sipavina L.V, Hafner S.S. (1995) Low temperature magnetic transitions in chain germanates. // Solid State Communications, V.95, N.6, pp. 405-408.
Nekrasov A.N. Development of the physical model for the magnetization of heterogeneous ferrimagnetic grains.
The development of the physical model for the magnetization of heterogeneous ferrimagnetic grains was initiated by the collaborators of the Analytical Laboratory, Institute of Experimental Mineralogy, Russian Academy of Sciences.
1. The spectral thermomagnetic analysis (STMA) technique was developed as a result of the co-operative work of the Analytical Laboratory, IEM, and Geomagnetic Laboratory, Physics Faculty, Moscow State University [1]. This method makes it possible to determine the distribution of the magnetic moments of the ferrimagnetic fraction of a sample by Curie temperatures provided the temperature dependence of the magnetization under the external field is known or the whole ferrimagnetic fraction is at the single-domain state.
The STMA technique was applied to trap samples from the Malo-Botuobinsk Region, Sakha-Yakutia Republic, and dredged samples of deep-sea basalts [1]. The spectral distribution of the chemical composition of the ferrimagnetic fraction of the samples was obtained from the compositional dependences of primary magnetic parameters (Curie temperatures and magnetic moment at T = 0 K) of the solid solution series Fe3-xTixO4. Physical and magnetic properties of hemoilmenite synthesized in the Laboratory of Metamorphism (A.N. Konilov) were studied [2, 3]. The possible application of the STMA technique to controlling changes in the physico-chemical composition of heterogeneous ferrimagnetic grains during their synthesis was shown. The application of the STMA technique in combination with nonmagnetic studies of ferrimagnetic samples (X-ray diffraction and X-ray spectral analyses) allowed the refinement of the compositional dependence of the spontaneous magnetic moment of hemoilmenite at absolute zero [3]. Nowadays the STMA technique is successfully used in a number of geomagnetic laboratories.
2. The theoretical examination of the magnetization of heterogeneous ferrimagnetic grains allowed the conclusion that the transition of these grains from the homodome to nonhomodome state is the I-type magnetic phase transition. It takes place if temperature or the external magnetic field
45
changes, appears as jumpwise increase or decrease of the magnetic moment of a grain, and is accompanied by absorption or exhalation of heat energy. The transition temperature decreases as the external magnetic field is enhanced and increases with the size and heterogeneity of the ferrimagnetic grains.
These theoretical conclusions were experimentally verified with an electrochemical nickel sample, hemoilmenite samples synthesized in the Laboratory of Metamorphism, IEM RAS, and rock samples containing titanom+agnetite with the use of the equipment of the Geomagnetic Laboratory, Physics Faculty, Moscow State University.
References:
- Ivanov A.P., Safroshkin V.Yu., Trukhin V.I., and Nekrasov A.N. (1992) Spectral Thermomagnetic Analysis of Rocks. // Fizika Zemli, no. 3, pp. 62-71.
- Trukhin V.I., Konilov A.N., Valeev Yu.K., and Nekrasov A.N. (1993) Hemoilmenite-Rutile Equilibrium and Investigation of Magnetic Properties of Synthetic Hemoilmenite. // Experiment in Geosciences, vol. 2, no. 4, pp. 2-14.
- Trukhin V.I., Nekrasov A.N., Konilov A.N., Zhlyaeva V.A., and Safroshkin V.Yu. (1995) Magnetism and Phase Composition of Hydrothermally Synthesized Hemoilmenites. // Fizika Zemli, no. 11, pp. 30-39.
Kalinichev A.G. Elastic and piezoelectric properties of ferroelectric single crystals KNbO3 and PbTiO3.
Brillouin-Mandelshtam light scattering technique was used to investigate elastic and piezoelectric properties of ferroelectric single crystals KNbO3 and PbTiO3 which have perovskite structure [1,2]. Optical absorption of PbTiO3 has been studied up to 350 kbar in the visible range of wavelengths using single crystal samples compressed in a diamond anvil high pressure cell. The absorption edge shifts towards lower energies with increasing pressure. Pressure dependence of the absorption edge undergoes significant changes at about 115 kbar, close to the previously identified tetragonal-cubic phase transition. The change in pressure dependence of the spectra is consistent with second-order character of the transition [1]. Apart from the geological importance of these results for the interpretation and modeling of the elastic properties of mantle minerals (many of them having the same perovskite structure), the new data are also of great value for many technological applications such as non-linear optics, pressure sensors, and other electro-mechanical transducers.
References:
- Zha C.S., Kalinichev A.G., Bass J.D., Suchicital C.T.A., Payne D.A. (1992) Pressure dependence of the optical absorption in PbTiO3 to 35 GPa: Observation of the tetragonal-to-cubic phase transition. // J.Appl.Phys., V. 72, pp. 3705-3707.
- Kalinichev A.G., Bass J.D., Zha C.S., Han P.D., Payne D.A. (1993) Elastic properties of orthorhombic KNbO3 single crystals by Brillouin scattering. // J.Appl.Phys., V. 74, pp. 6603-6608.
47
Previous
Contents
Next
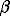 -quartz on a seed was developed. The results obtained enable the systematic experimental study of the properties and physicochemical conditions of
-quartz on a seed was developed. The results obtained enable the systematic experimental study of the properties and physicochemical conditions of 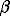 -quartz origin in nature.
-quartz origin in nature. 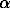 -
- H(
H(