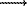 chalcopyrite
chalcopyrite 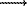 sphalerite
sphalerite 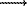 galenite.
galenite.III. Metasomatic processes and modelling of the ore formation
Zharikov V.A. and Zaraisky G.P. A numerical model of the tungsten greisen deposit genesis, (with the example of the Akchatau Deposit, Central Kazakhstan).
The results of long-term investigations of formation of Akchatau W-Mo greisen deposit, Central Kazakhstan, were summarized (Zharikov Zaraisky, 1995). The time of complete crystallization of the Akchatau pluton was determined as about 570 ka on the basis of the correlation of geological, experimental, and numerical computer modelling data. The temperatures of magma crystallization, greisens and quartz ore veins formation was determined from oxygen isotope and experimental modelling data, whereas the initial and final temperatures of each hydrothermal stage of mineral and ore formation were established from fluid-inclusions data. The detailed geochronology scheme of the deposit origin was first established from the correlation of calculated evolution of the thermal field of the Akchatau intrusion with independently determined temperatures of formation of granites, pegmatites, greisens, and ores. Greisen formation (550-350oC) occurred during the early consolidation for the leucocratic granite pluton, 200-270 ka after the main pluton was intruded. The quartz vein and rare-metal ore formation (490-260oC), including W, Mo, Be, Sn, and Bi, occurred later after the beginning of greisenization (no more than 20 ka), but it was completed before the intrusion crystallization: 220-400 ka. However, the whole hydrothermal process (490-150oC) occurred in the interval from 220 ka to 1 Ma, which is beyond the time of the magmatic chamber activity.
The late polymetallic galena-sphalerite-quartz stage and final post-ore fluorite-quartz and zeolite-calcite hydrothermal stages are assigned to the post-crystallization period of the system evolution. It should be noted that at the end of the granite melt crystallization, the concentrated F-Cl-Na-K fluids change to dilute H2O-CO2-NaHCO3 solutions. Isotope analyses of the host granite, metasomatites, and ores of the deposit have shown that it was formed by the magmatic fluid of the source granite. The fluid isotopic composition equilibrated as the intrusion cooled, and the meteoric water convection in the cooling intrusion occurred only at the final stages.
Numerical modelling of thermoelastic stress and deformations in the granite body and host rocks indicated that the main ore-controlling subvertical fractures of Akchatau are genetically related to thermal field evolution at early stages of cooling and crystallization of the granite pluton. These fractures resulted from thermogradient stress, occurred at the early crystallization stage (from 0.0 to 0.2 of crystallization period) and are confined to the peripheral parts of the pluton (to a depth of 0.25 of estimated radius).
The quantitative analysis of tungsten transport and deposition at the thermal barrier by regular ascending flow of magmatogene water-bearing fluid showed, that the formation of large deposit (such as Akchatau) with W-reserves about 100 thousand tons requires the focused filtration of the whole bulk of magmatic fluid that would resulted from the crystallization of granite melt block 20 km2 in area and 8 km thick. These data are consistent with the size of the Kyzyltas granitic dome, which hosts the main ore bodies of Akchatau.
References:
24
Ezhov S.V. and Zaraisky G.P. Skarns and skarn formation.
The physical-chemical conditions of formation of epidope bimetasomatic skarns typical of the Altyn-Topkan lead-zinc deposit (Mid Asia) have been experimentally studied and possibility of simultaneous deposition of Pb, Zn, and Cu at the formation of scarn zones has been shown (Ezhov, Zaraisky, 1993). The temperature range of the epidope scarns formation was found to be 350-550oC. Their formation is favoured by the enhanced potassium content in the starting granitoids and low (NaCl+CaCl2)/KCl ratio in the affecting solutions. Epidope scarn facies can be indicators of low partial CO2 pressure corresponding to the mole fraction of CO2 to 0.04 at PH2O+CO2=1 kbar.
The ore metals (Pb, Zn, Cu) when introduced into the solution in concentrations of saturation in galenite, sphalerite, chalkopyrite deposited at the contact of the zones of bimetasomatic columns with the simultaneous formation of the latters (Ezhov, Zaraisky, 1994). Pyrite is the first to appear due to iron of dark coloured minerals of granodiorite-porphyry, then the edging accretions of chalcopyrite appear, i.e. it is a precipitator of copper. Lead and zinc diffuse in the pore solutions through granodiorite-porphyry smoothly and precipitate in the scarn zones near the contact with limestone apparently at the alkaline barrier. In the direction from granitoid to limestone, ore minerals deposit in a certain sequence: pyrite 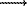 chalcopyrite
chalcopyrite  sphalerite
sphalerite  galenite.
galenite.
A notable effect of sulphate sulphur present as an anhydride on the intensification of the scarn- and ore-formation processes was shown experimentally (Ezhov, 1996). The conjugate iron oxidation to three valence state and reduction of sulphur to sulphide-ion favours the simultaneous development of scarn minerals containing three valence iron (andradite, epidope) and ore sulphides Pb, Zn, and Cu. The local increase in sulphide sulphur concentrations in the zones of the andradite and epidope scarns formation, provide the conditions for the deposition of galenite, sphalerite, and chalcopyrite from the pore solutions with low concentrations of ore metals. The hypothesis of mutually conditioned formation of scarn and ore minerals seems to be perspective for the elucidation of the genesis of some types of scarn deposits.
The experiments on the transformations of ore aggregates for the first time visually showed how fast and deep under the effect of temperature could be the recrystallization of sulphide minerals (sphalerite, galenite, chalcopyrite) commixture. It takes some tens or first hundreds hours that is, in geological measure, an instant (Ezhov, 1996). Herewith, this time is enough not only for shape and size of mineral grains to change drastically, but sometimes for the ore aggregate to change its texture from the homogeneous to zonal banded one. After the experiment ore minerals initially being homogeneous mixture segregate as independent bands. It is important for the further investigations that the motive force of this surprising "selforganization" could be such an inessential gradient of chemical conditions which arouse due to the contact of ore minerals mixture with different rocks: quartz porphyry on the one hand and granodiorite-porphyry on the other hand.
References:
Zaraisky G.P., Aksyuk A.M. , Korzhinskaya V.S. . Korzhinskaya V.S. Experimental study on the behaviour of rare metals, polymetals, silicon and aluminum in fluoride and chloride hydrothermal fluids.
The effect of acid fluoride solutions on the geochemical behavior of Si, Al, and W at greisen formation has been studied. It was found that at really possible HF concentrations in natural solutions (to 0.1 m) the presence of F only insufficiently increases Si and W mobility. However, a notable migration of usually inert Al at greisenization may be explained by the effect of acid fluoride solutions with the concentration HF=10-3-10-1m, in which Alaq increases
25
from 10-4to 10-2 , i.e. almost reaches the solubility of silica at the same P-T parameters. As for silica, no problems of mass transfer are known at hydrothermal conditions. Based on experimental data on the corundum solubility, the free energies of formation of aluminum hydroxofluorine complexes and concentrations of 7 major Al-bearing particles of acid fluoride solution in equilibrium with corundum were first determined at T=300, 400, 500, 600oC, P= 1 kbar. It was established that in the range of starting HF concentrations from n*10-3 to n*10-1 mol/kg H2O, the neutral hydroxofluorine complexes Al(OH)F2o, Al(OH)2Fo and aluminum fluoride AlF3o dominate in the solution. At lower HF concentrations (10-4-10-3m) hydroxocomplexes and Al3+ion become the major Al-bearing particles. Acid chloride solutions cannot provide alumina mobility because in this case unreally high for natural conditions HCl concentration (above 0.1m) would be needed (Zaraisky, Soboleva, 1992).
Experimental study on wolframite solubility in fluoride solutions showed that equilibrium tungsten content in the solution remained practically so as in the pure water up to the HF concentration of 1.0 m and increases only with the further increase in the concentration. This suggests that fluoride particularity of the solutions at the greinisation cannot be a determining factor of the tungsten transfer and deposition, so as it does in relation to alumina (Zaraisky, 1995).
The experimentas were carried out and the data were obtained on the fluorine distribution between phlogopite and fluid at T= 500-700oC and P= 1-4 kbar. Based on the above results the first in the world phlogopite geofluorimeter has been developed which allows to estimate real fluorine concentrations in ancient mineral-forming fluids by phlogopite composition, temperature and water activity.
lg M(HF) = X(Mg)[lg(X(F)/(1-x(F))]-1722/T-0.2112+lg aH2O.
This relationship and experimental data of Munoz (Munoz, 1984) allowed to develop a biotite geofluorimeter for the estimation of the HF concentration in a wide spectrum of processes of mineral formation in different geological environments. It was tested at some granite plutons of Kazakhstan and porphyry-copper deposits. It was, in particular, shown that copper-porphiti deposits of the Ak-Sug, Erdenet type are characterized by almost an order lower HF concentrations than greisen deposits of the Akhchatau type (Aksyuk, 1995).
The equilibrium ferberite-sheelite was experimentally studied in chloride solutions at T=300-600oC in the presence of oxygen buffers Ni-NiO and Fe-FeO. A distinct positive dependence of the reaction of ferberite substitution by sheelite on temperature and less distinct positive dependence on oxygen fugacity. A linear character of concentration equilibrium constant was established only for high temperature and reducing conditions (600oC, Fe-FeO buffer). Thermodynamic processing of experimental data using the "Gibbs" software allowed to rifine the free Gibbs energy for ferberite and sheelite (Korzhinskaya, Zaraiskii, 1997).
Experiments were conducted on the gain and loss of base metals (Cu, Pb, Zn) under the conditions of interaction of leucocratic Akhchatau granite with the solutions of different composition at T=400oC, P=1kbar. Copper and lead were found to show notably higher migration ability than the zinc: Cu>Pb>Zn. The three metals easier migrate in chloride solutions than in fluoride and alkaline ones. This is their principle distinction from W and Mo, for the transfer of which the alkaline and fluoride conditions are most favorable. Under the conditions studied, Cu, Pb, and Zn transfer proceeds easier and further along the column (up to 20-48 mm in two weeks) than that of W and Mo ( not more than 10 mm in the same period). Only Sn transports up to the depth of 20-25 mm in the solutions Na2SiF6 and NaCl. Base metals when gained show somewhat higher concentrations in metasomatic columns than W, Mo, and Sn. In the ‘weight loss experiments base metals, in particular copper, often redeposit in the intermediate and advanced zones of metasomatic columns in the amounts exceeding the starting content by 500-3500 ppm. Rare metals do not show this feature (Zaraisky, Stoyanovskaya, 1995).
References:
#Shapovalov Yu.B. Experimental study of rare and rare earth metals and base metals behaviour in the fluid-magmatic granitoid system.
The process of ore concentration of tungsten, tantalum, niobium and tin was experimentally studied in the system granite-fluorine fluid at 960oC and 1 kbar. Petrogenic and ore metals were found to behave differently under salt extraction. In experiments silicate glasses concentrated silicon and potassium, while the Si-poor salt phase, was enriched in sodium, calcium, and magnesium. Tungsten is extracted and concentrated intensively in the salt phase (especially by LiF), whereas Ta, Nb, and Sn are indifferent to it and are retained in the granitic melt (Marakushev, Shapovalov, 1994).
#This study was supported by the RFBR, Grant No. 95-05-14840
26
The distribution of rare-earth elements between silicate and salt phases as a result of liquid differentiation was studied. It was established that salt phases are effective concentrators of rare-earth metals and sodium, whereas potassium is concentrated in aluminosilicate melts. All salt phases are very poor in aluminum and can be divided by Si content into salt and silica-salt types. The latter are up to 30% SiO2 and formed in those runs, where silicon was a constituent of salts (K2SiF6 and Na2SiF6). Light (La,Nd), intermediate (Gd) and heavy (Dy, Lu) rare-earth elements are equally well concentrated in the salt phases. A markedly higher lanthanium concentration compared to heavier metals was established in the salt melt of the granite-LiF system (Marakushev et.al, 1994).
The partition coefficients of ore metals (Mo, Pb, Zn) between the coexisting immiscible granite aluminosilicate and fluorine-salt melts were determined at T=960oC and P=1 kbar. The marked enrichment of the salt melt in Mo and Pb was established. The granite/salt partition coefficients were determined for Mo (up to 0.03), Pb (0.10), and Zn (about 1.0). The partition coefficient of Zn decreases to 0.29 in the system with CaSiF6, but nevertheless it is much higher then that for Mo and Pb in the same system (Marakushev, Shapovalov, 1996).
References:
Chevychelov V.Yu. Partitioning of W, Mo and Pb, Zn in the fluid-magmatic granitoid systems. Effect of the melt composition.
The effect of the composition of the model granitoid melt on the process of concentration in it of some rare metals (W, Mo) and base metals (Pb, Zn) in the presence of chloride and fluoride bearing acidulous water fluid has been studied at T= 800-1000oC and P= 1 and 5 kbar. The certain differences in the partitioning of metals between fluid and melt were determined at the decrease of the melt basicity in the series granodiorite- granite- leucogranite.
No notable differences in the behavior of W and Mo were observed in the chloride bearing systems. Our data show rather low (200-500ppm) contents of these metals in the melt composition. At P=1 kbar an increase of the partitioning coefficient (KD=Cfl/CL) of rare metals between a fluid of the 1m NaCl + 0.1m HCl composition and granitoid melt in the granodiorite- granite- leucogranite series has been experimentally found to be 0.2-0.3 to 0.7-0.9. The supposed reason of this increase is the change in CaO content in the melt which keeps W and Mo in the form of scheelite-powellite component. The effect of the melt composition becomes less with pressure: at P=5 kbar KD is 1.0-2.0 for granodiorite and granite and 2.0-2.7 for leucogranite (Chevychelov, Chevychelova, 1997).
If fluoride fluid (0.2m HF) is present in the system instead of chloride one the content of W and Mo in the melt increases to 1000-1600 ppm at P=1 kbar. Under such conditions the difference in the partitioning of these metals becomes evident. So, KD of tungsten and molybdenum are respectively, 0.35 and 0.1 for granodiorite, 0.4 and 0.14 for granite and 0.8 and 0.2 for leucogranite. The values of KD markedly grow with pressure but the influence of the melt composition is inessential. At P= 5 kbar, KD of tungsten changes depending on the melt composition within 1.9-2.7 and KD of molybdenum changes within 2.1-3.7 (Chevychelov, 1996).
For lead and zinc the partitioning maximum in favor of the fluid was determined in case of the granite and leucogranite composition. It was found that partitioning coefficient of these base metals increased with decreasing basicity of the melt (growing the silica content) in the basalt-andesite-granodiorite-granite-leucogranite series. The results are the values intervals obtained in the course of experiments by the method of "approaching the equilibrium from two sides": from melt and fluid. KD of zinc is in average 1.5-2 times higher than that of lead. At P=1 kbar in the granodiorite-granite-leucogranite series it is 1.2-2.1; 3.0-7.1; 3.1 for zinc and 0.7-2.1; 2.9-3.3; 1.6-3.2 for lead. KD of these metals become somewhat lower with increasing pressure to 5 kbar (Chevychelov, Chevychelova, 1997).
References:
#Redkin A.F. Solubility of cassiterite and uranium oxides UO2+x at T=600oC and PH2O= 1000 bar.
1. The weight loss method has been used for an investigation of cassiterite SnO2 (natural, Vietnam) solubility in the 0.1 mHF + n mNa2B2O7 × 10H2O, where n= 0, 0.125, 0.08 and 0.25, as well as in 0.025 and 0.25 m borax solutions at 500oC, 1,000 bar, Ni/NiO oxygen buffer and run duration of 14 days. It was found that SnO2 solubility has tendency to increase with concentration of borax from 10-3.03 (pH25=2.84) to 10-2.06 (pH25=9.20) mole/kg H2O. The results of the runs are shown in Fig. 1.
#This study was supported by ISF and the Government of Russian Federation grant N5F000.
27
Three control kinetic experiments on synthetic SnO2 crystal solubility were performed in a large tube of 20 cm3 in the solution of 0.1mHF+0.0125m Na2B4O7 ×
10H2O and run duration of 9, 14 and 80 days. The results are as follows (log mSn/days): -2.34/9, -2.49/14, -2.26/80. The average value is -2.35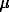 0.10. It is 1-2 order higher than that received by Kovalenko et al. (1991) by quench method.
0.10. It is 1-2 order higher than that received by Kovalenko et al. (1991) by quench method.
If proposed that the mass transfer of tin from crystal of SnO2 to Pt tube is negligible then this high cassiterite solubility sould be involved in borate fluoride complexes of tin. The obtained results may be used for explanation of tin geochemistry in the ore accompanying stage of greisen process having a boron fluorine character.
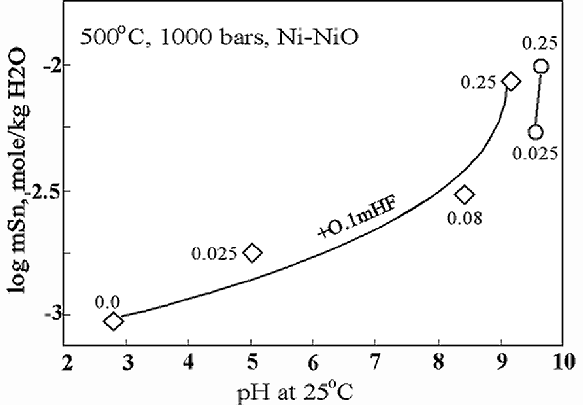
Fig.1. SnO2 solubility in borax (round symbols) and in hydrofluoric acid-borax solution (rhombis). The values at the symbols are the molality of borax
2. The solubility of UO2 and other uranium oxides and hydroxides has been investigated at temperatures 200-600oC and pressure of 1,000 bar in f(O2) buffered systems and in different hydrothermal solutions. It was necessary to clear up the behavior of uranium in the magmatic and post magmatic processes. As a result of the investigation it was found that
-the UO2 solubility in pure water in the temperature range 300-600oC and f(O2) from Ni-NiO to Fe2O3-Fe3O4 buffers is about 2 × 10-9 mol/kg H2O;
-the solubility of UO2 at Ni-NiO buffer is described by equation:
in HCl solutions of 10-4-1.0 mole/kg H2O at T=500oC: mU=10-3 × mHCl;
in chloride solutions (mCl=mKCl+mNaCl+mHCl @ 1 mole/kg H2O):
at 400oC mU = 10-1.8 × m2HCl+10-6,
at 500oC mU = 10-2.0 × m2HCl+10-6,
at 600oC mU = 10-3 × mHCl +10-6.
-the following relationship of UO2 solubility in aqueous HF solutions at 500oC takes place:
at Ni-NiO mU = 2.353 × 10-2 × m2HF +8.293× 10-4 × mHF +10-6,
at Fe2O3-Fe3O4 mU = 0.1158× m2HF +3.207× 10-2 × mHF +10-6.
-UO2 solubility in aqueous solution of 0.5mNaOH+0.25mNa2C2O4, 0.5mNa2CO3, and 0.1mH2C2O4 averages 3× 10-6 mol/kg H2O at 500oC.
-the data on U(VI) hydroxides solubility in pure water in the temperature range from 200 to 500oC can be described by the equations:
log mU = 15375.3/T + 0.0647× T -67.05
200oC£ T-273.15£ 300oC,
log mU = -221.7/T -2.71300oC£ T-273.15£ 500oC.
Based on the hydrothermal experiments of using an Ag-AgCl sensor procedure, it was determined that equilibrium oxygen fugacity of U3O8-UO3 × 0.33H2O buffer assemblage is much the same that as of f(O2) of Cu-Cu2O oxygen buffer at 400, 500oC and H2O pressure of 1,000 bars and is -18.1 + 0.6 and -15.0 + 0.4 in terms of log f(O2), respectively. The data obtained are not in contradiction with the natural mineralogical observation but they are in a rather poor agreement with the thermodynamic calculations.
The obtained data are in agreement with the notion of magmatic nature of uranium ore solutions and possibility of U(IV) migration both in Cl- and F- hydroxide complexes. (Redkin, 1993, 1996).
References:
Shmonov V.M. Investigation of rock permeability in the tense states at high parameters.
The following apparatuses were developed, assembled and brought in operation to carry out experimental study and determine total permeability of a rock as well as contribution of crack and pore components with account of pore size and morphology of pore space at high parameters, tectonic (deviation) stresses, chemical composition of the solution and other factors.
"Deviator-3" - for determining permeability of the rock samples 0.25 x 40mm in size at the conditions of inequality of stresses included along the three axes at total pressure to 2000 kgf/cm2 and temperature to 600oC.
"Micron-600" - for searching for the cracks development at the sample heating to 600oC immediately in the electron microscope.
"Angstrem-1000/600" - is also used in the electron microscope at temperatures to 600oC and pressure on the sample 1000kg/cm2.
28
"Galit-10/800" - to determine rock permeability at pressures to 10 kbar and temperatures to 800oC; is used in the piston cylinder apparatus (jointly with Prof. Perchuk and Dr. Ishbulatov).
"Standart" - to determine permeability at the standard conditions, i.e. at room temperature without confining pressure.
A cell for immediate observation of the sample structure in the scanning electron microscope at T=600-750oC and P to 1000 bar has been developed. The experiments were conducted on the study of cracks distribution by sizes in basalt at T to 750oC without loading at the samples. Cracks do not form under experimental conditions at the heating rate of 3 degrees per min as well as at sharp temperature decrease (about 100oC/min). Microcrack permeability of basalt calculated on experimental results is from 50-500mDarcy to 110-730 mDarcy at T=200-600oC. As it was expected microcrack permeability of rocks is several orders higher than the pore one.
Using this experimental equipment the permeabilities of oceanic basalts, gabbro and serpentinites of submarine lava flows from different depths, of different compositions and age were measured at T=20-600oC and P= 300-1500 kgf/cm2. The range of basalts permeability was found to be 10-16-10-24 m2. It was established that basalt permeability depends on their age (the most ancient basalts become less dense with temperature) and on the degree of their serpentinization. At T above 200oC highly serpentinized rocks become virtually impermeable . Permeability of gabbro and hyperbasites at their starting porosity of 0.71-4.71% (which is 2-3 times higher than that of serpentites) is 10-15m2. This is by an order higher than permeability of basalts and by several orders higher than that of serpentinites. Computer programs (BAISIK) were developed for the processing of observation results. They allowed to calculate permeability of rocks by transient pulse method, method of end differences and determine an effective pore radius. The results obtained using above programs are presented in (Shmonov et al., 1995).
Reference :
Balashov V.N. , Ivanov I.P. Theoretical modeling of metasomatic zoning, topological analysis and thermodynamic calculations of mineral equilibria.
A qualitative mathematical model and computer software EHS-2 has been developed for the calculation of infiltration metasomatic zoning at the interaction of a solution of given composition with the initial rock under the conditions of local equilibrium. Using the program the computer modeling of zoning was performed in the simplified granite system K2O-Na2O-Al2O3-SiO2-H2O-HCl. The numerical algorithm of the calculation of infiltration metasomatic zoning with a phantom zone involving all the possible cases of zone-phantasm appearing in the column was theoretically substantiated and developed. Local-equilibrium investigation of the zone-phantom in the multicomponent systems has been completed. The algorithms and the program were developed on the calculation of infiltration metasomatic zoning in the common case with the zones-phantoms in the multicomponent systems. The algorithms of plotting the asymptotic curve in time for the diffusive-convective metasomatic zoning in the full kinetic light were developed. It was proved that asymptotic curve in time of the solution of equations describing the advancement of zoning front at the complete modeling ( with the kinetics of reversible reactions) is a solution of the "running wave" type.
The calculations of the solubility diagrams in the end system Al2O3-SiO2-H2O-HCl (in pure water in the 10-1m HCl solution) at T=200-400oC and P=1kbar were carried out. The T-P diagram of this system which displays all the types of facial zoning and allows to estimate the evolution of of local-equilibrium process in space and time (telescoping) was refined . The compositions of the solutions equilibrium with minerals of internal and external zones in the columns of Si-metasomatites were given. It was shown that quartz solubility in weakly acid solutions is close to that in pure water. Silica solubility, at the contrary, notably depends on pH of the solution. In acid solutions its solubility is retrograde and increases by 2-3 orders below 300oC. To prove this conclusion the experiments in diaspore solubility at T=400-200oC and P=1kbar were carried out. It was shown that convergence of mineral zoning in the solutions with HCl and H2SO4 is explained by the solution acidity only.
A complete topological analysis of T-P equlibria in the "model" system Na2O- K2O- Al2O3- SiO2-H2O was done. In the range T=25-550oC and P=1 kbar the reactions equlibria were calculated, the parageneses related to hydrolysis in the boundary sodium system and stability of AuOH hydrocomplex in the chloride and sulfate (sulfide) solutions were studied at T=200-400oC and P=1 kbar (Ivanov, 1995).
References:
29