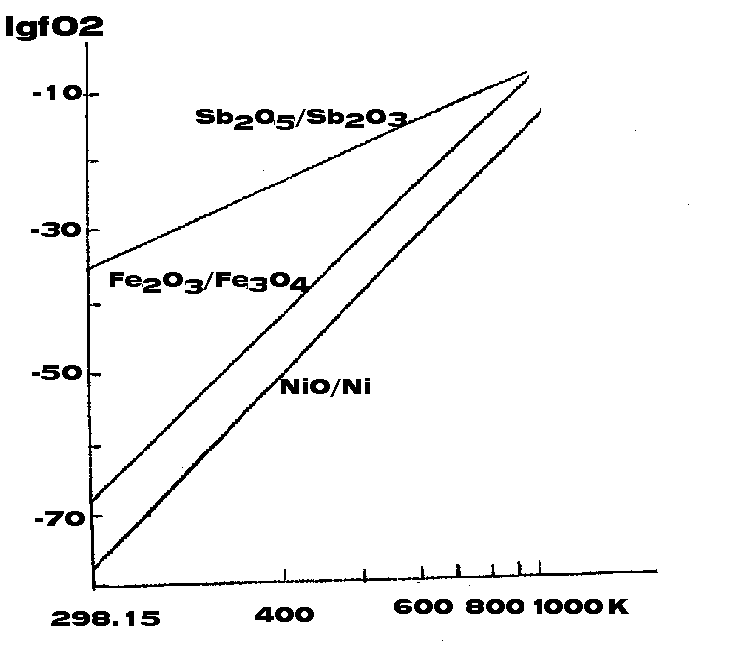
Abstracts. Khitariada-96
Magmatic systems, fluid magmatic
interaction, melts properties
#
Khodorevskaya L.I. Experimental study of the amphibolite-lherzolite interaction and its application to subduction zones.key words [amphibolite lherzolite fluid magma]
Subduction of the oceanic crust results in rock melting because of dehydration of water-bearing minerals. The rising fluid flows originating from the dehydration of the subducted block can alter the overlying more heated mantle rocks by adding silica and other components.
The compositions of fluids and quenched glasses simulating the natural melts and minerals were investigated. The experiments present the primitive model of the interaction of hot mantle rocks with subducted colder rocks of the oceanic crust.
The experiments were conducted according to the following scheme: lherzolite sample (olivine, clinopyroxene, orthopyroxene, and chromospinelides) (25 mg) and amphibolite sample (amphibole and plagioclase) (900 mg) were placed in the upper and lower parts of the capsule respectively. The excess of amphibolite over lherzolite provided the buffer capacity of the fluid with respect to dissolved components. Distilled water was added, and the capsule was sealed. The fluid/solid ratio was (1-3) : 1. The lower part of the capsule was heated to 700, 750, 800, and 850oC, and the upper one was heated to 990-1000oC. Temperature was controlled with a Pt/Pt (Rh 10 wt %) thermocouple. The runs were conducted at 8 kbar for 3 days. Oxygen fugacity was not controlled, because the fO2 effect on the melt composition is slight at P = PH2O in the amphibole field, according to Helz (1976).
The main phases formed on the lherzolite were amphibole and melt. Clinopyroxene and olivine in the lherzolite were preserved but their composition changed during the runs. The composition of the quenched glass formed on the lherzolite is determined by the amphibolite solubility at various temperatures and the fluid impact on the lherzolite. The melts obtained are similar in petrogenic component contents to intermediate rocks (Si about 60%, Al 20%, Ca 10%, Fe 1-5%, Mg 1-4%). The forming fluid phase is represented for the most part by Na = 0.2% and Si = 0.1-0.2%.
So, in the continent-ocean transition zones, the rocks of mantle wedge are evidently altered by the fluids of significantly Na and Si composition that originate from the dehydration of the subducted oceanic crust rocks. The melt close to andesite in composition was formed on the lherzolite in our experiments. On the background of partial melting of the ultrabasic rock, the formation of high-magnesian aluminous amphibole is possible with its subsequent melting to yield high-magnesian magma. The presence of Na in the fluid that forms, for example, on decompression can enhance the fluid aggression and cause the extraction of ore components from solid phases by the fluid and their transportation..
Kholodnov V.V., Bocharnikova T.D., Chashchukhina V.A., and Kraeva Yu.P. Halogen role in fluid-magma interaction in basic magmatic systems (Magnitogorsk iron ore field).
key words [ chlorine fluorine basic rocks]
The behavior of petrogenic and ore components was studied within the vertical section of the subvolcanic porphyritic intrusion (thickness about 1000 m) at the under side of the Magnitogorsk skarn-magnetite deposit. Two different trends of component distribution were established. The contents of some components vary jumpwise (at SiO2 50-52%) when passing from the pyroxene porphyrites of the intrusion base to the pyroxene-plagioclase and plagioclase porphyrites of the middle part and then to the trachytes (atachites) of the roof. The atachites, representing the upper horizons of the intrusion, are enriched in titanium, phosphorus, and alkalies, while the pyroxene porphyrites of the base are enriched in magnesium and calcium. The pyroxene-plagioclase and plagioclase porphyrites are characterized by intermediate contents of the components. As can be seen from Fig.2, Accumulation of alkalies is accompanied by the separation between sodium and potassium to yield sodium-rich and potassium-rich varieties of atachites, plagioclase porphyrites, etc. Potassium contents correlate with rubidium and some other trace elements (La, Ce, Th, etc.). In turn, the contents of the components vary
# This study was supported by the Russian Foundation for Basic Research, project no. 96-05-64871
10
gently within each of the aforesaid rock types: in pyroxene porphyrites SiO2 44-52% and in the rest two types SiO2 50-60%.
The superposition of these regularities is probably an evidence for complicated evolution of the intrusion. The concurrent gradual decrease of Mg and Ca contents and increase of SiO2, K2O, and Rb contents upward the section obviously is a sequence of the crystallization differentiation. However, the discrete changes of the component contents within the range SiO2 50-52% are likely to result from the differentiation of any other type and call for special investigation.
In this connection, we studied in details the behavior of halogens in the vertical section of the intrusion. It was established that the pyroxene porphyrites of the intrusion base contain the most Cl-rich apatite, while the upper more acidic rocks are depleted in chlorine, but enriched in fluorine. Thus the tendency was revealed that was previously established for a number of classic layered basic-ultrabasic intrusions (Stillwater, Boushveld, etc.).
Three main phases of the halogen evolution were distinguished. The first phase corresponded to the formation of the pyroxene porphyrites of the intrusion base. The crystallization was accompanied by a sharp decrease of chlorine content in apatite from 2-2.8% to the level of Cl/F ratio equal 0.9. The subsequent two phases are manifested in the overlying rocks. The second phase was charaterized by gradual increase of chlorine and fluorine contents in apatite at constant Cl/F ratio upward the section (from the pyroxene porphyrites to the pyroxene-plagioclase and plagioclase porphyrites and atachites). The third phase involved crystallization of these rocks and is accompanied by further chlorine loss and fluorine accumulation.
These data testify that the phorphyritic intrusion under study was alternatively open or closed fluid-magmatic system at different phases of its evolution. The sharp decrease of chlorine and increase of fluorine contents can result from the volatilization of chlorine and water vapor from the melt and respective fluorine accumulation, which are dictated by the constants of partition of Cl, F, and H2O among fluid and melt (40 for chlorine, 0.50 for fluorine, and 0-20 for water). It is usually assumed that the separation of volatiles from melt during crystallization (open systems) is caused by either decrease of the external pressure or increase of the fluid partial pressure. The model involving crystallization and fractionation of the minerals free of volatiles (olivine, pyroxene, plagioclase) charaterizes the general increase of fluid phase content in residual melt. In this case, no significant volatile fractionation takes place, and the Cl/F ratio in apatite is close to constant (closed systems). This model can describe the further accumulation.
These data testify that the phorphyritic intrusion under study was alternatively open or closed fluid-magmatic system at different phases of its evolution. The sharp decrease of chlorine and increase of fluorine contents can result from the volatilization of chlorine and water vapor from the melt and respective fluorine accumulation, which are dictated by the constants of partition of Cl, F, and H2O among fluid and melt (40 for chlorine, 0.50 for fluorine, and 0-20 for water). It is usually assumed that the separation of volatiles from melt during crystallization (open systems) is caused by either decrease of the external pressure or increase of the fluid partial pressure. The model involving crystallization and fractionation of the minerals free of volatiles (olivine, pyroxene, plagioclase) charaterizes the general increase of fluid phase content in residual melt. In this case, no significant volatile fractionation takes place, and the Cl/F ratio in apatite is close to constant (closed systems). This model can describe the further accumulation of halogens in the melts forming the roof of the intrusion.
The studies performed revealed the latent layered structure of the intrusion that existed prior to its crystallization. On the background of the layered structure, the lighter fluid enriched in fluorine ascended to the upper horizons of the intrusion, and the heavier fluid was concentrated at the base. Simultaneously, all the elements forming compounds with fluorine (potassium, silicon, aluminum, rubidium, titanium, phosphorus, etc.) migrated upwards as a result of the fluid-magma interaction. The jumpwise zonality of petrogenic and volitile components within the range SiO2 50-52% can be considered as a result of the evolution of originally uniform basalt melt.
The iron distribution in the three rock types is specific. The chlorine volatilization during the magma crystallization makes it possible to consider the porphyritic intrusion as a possible source of iron-bearing ore-forming fluids. This assumption is supported by the pyrite-magnetite mineralization confined to the contact of the atachites with limestones. Thus the problem of polygenic character of the skarn-magnetite mineralization of the Magnitogorsk deposit arises. The formation of the ore field was then continued during the formation of the Magnitogorsk gabbro-granite intrusion and post-intrusive sill-dike gabbro-diabse complex.
# Kravchuk I.F., Malinin S.D., and Slutskii A.B. The principle factors of ore element fractionation in the acidic magmatic process: experimental data.
key words [granite melt water salt fluid fluid ore]
Studies of the ore deposits associated with hot springs and fumaroles showed the magmatic fluids to be the source of the ores. Definite genetic relations between magmatic objects and hydrothermal deposits were established. However, the widespread hydrothermal activity results in formation of the ore deposits only in rare cases.
Thus, determination of the factors setting off the potential ore-bearing hydrothermal systems is a challenging problem.
The method of studies involves the experimental simulation of element distribution among the phases in the system melt-fluid at the acidic magmatism parameters.
The objects of investigation: natural granite and its simplified artificial counterpart; water-salt fluids (NaCl, KCl, and HCl solutions, their mixtures, and
# This study was supported by the Russian Foundation for Basic Reasearch, projects nos. 94-05-17443 and 95-05-15068.
11
NaF solutions); elements: Zn, Cu, Mo, and W (typical elements of hydrothermal magmatic ore deposits).
The experimental equipment used: 3 types of high-pressure apparatus: high-gas-pressure vessel with internal heater, double-chamber apparatus with external heater, and the apparatus of piston-cylinder type.
Main conclusions:
1. The partition constant Ki=xifluid/ximelt in the system Ab-Or-Qz-1m NaCl-solution (model granite system) decreases in the series
Cu - Zn - W - Mo Ki >>1 >1 >1 >1
at T = 800oC and P = 2 kbar.
2. Copper and zinc form complexes with Cl-; complexation of tungsten with Cl- is very probable; molybdenum is more likely to form complexes with F- and hydroxyl rather than with Cl-.
3. Pressure increase results in decreasing KZn and KCu (to a lesser degree) and increasing KMo. The data on W are to be verified.
4. Temperature increase results in increasing KMo (KW ?) and decreasing KCu and KZn.
5. The molecular ratio Na+K/Al has a significant effect on Ki (i=Cu, Zn): the Ki values increase by a factor of ten when passing from Na+K/Al>1 to Na+K/Al<1.
6. KCu and KZn increase with increasing fluid acidity.
7. Heterogenization of the fluid results in marked enrichment of the salt fluid phase (fl.1) with ore elements compared to the significantly aqueous fluid phase (fl.2). At T = 800oC and P=1 kbar: K'Cu=14, K'Zn> 5, and K'W=10-12, where K'i=xfl.1/xfl.2.
Geochemical application
The fluids of different ore potential are separated out as a result of crystallization of granitic magma, depending on crysallization conditions. For example, pressure decrease enhances fractionation of Cu, Zn, (W ?) in favor of the fluid and results in decreasing KMo. Further concentration of these ore elements in the salt fluid phase occurs as pressure decreases to the value corresponding to the fluid heterogenization. The melt composition, especially Na+K/Al ratio, is responsible for Ki values: ore-bearing fluids (Cu, Zn) are likely to originate from Al-rich granites at shallow depths (P<1-2 kbar). The effect of pressure on the Mo fractionation is opposite to that on Cu and Zn, while as to W, this effect is of no importance. The Mo-W deposits are expected to form at great depths, with the fluid heterogenization appearing as a principle factor responsible for the ore-bearing fluid formation.
The partition constants of the elements yielding complexes with Cl (Cu, Zn, and W) depend on chlorine concentration in the solution and, consequently, on chlorine content in granite. The recent studies of melt inclusions in acidic rocks (Naunov et al., 1994) showed high chlorine contents (up to 0.2-0.3 wt %), which are suffucient for metal transfer, according to Nakano and Urabe's (1989) evaluation (200-1000 ppm Cl).
The water/chlorine ratio is an important factor of fluid evolution. The fluids with high Cl/H2O ratio are most effective as to transfer of ore elements. Equilibrated with the melt containing 0.1 - 0.3 wt % of Cl-, such fluids can contain up to 50 wt % of chlorine in the form of alkali metal chlorides (Kravchuk and Keppler, 1994). The association of molybdenum minerals with fluorite seems to be regular, when considering the high probability of Mo transfer in the form of fluoride complexes and the inverse dependence of KMo on CaO content in the melt established before (Malinin and Kravchuk, 1991). The reactions of crystal-melt type, which can also play a significant part in formation of ore-bearing fluids (Ryabchikov, 1975), are not considered in this study. As a result of such a distribution, tungsten and molybdenum are always accumulated in fluid, and Zn2+ and Cu+ show limited solubility in granite minerals (feldspars, anorthite), which further drops with decreasing temperature.
Durasova N.A., Kochnova L.N., Khramov D.A., Slutskii A.B., and Troneva N.A. Antimony in alumosilicate glasses of granite eutectic composition.
key words [antimony melt fluid granite]
The Sb-bearing alumosilicate systems of granite eutectic composition were experimentally studied at liquidus and subsolidus temperatures and variable redox regime to establish the conditions favorable for extraction from igneous rocks and concentration of antimony and other polyvalent elements. The experiments on interaction of acidic solutions with Sb-bearing glasses were also performed.
Our previous calculations of equilibrium oxygen pressures in the system reduced form - oxidized form for a number of ore elements, including antimony, [1] (figure) showed that antimony in different states of oxidation can exist at fO2 of natural systems. The polyvalence of antimony is responsible for its geochemistry and behavior in magmatic and post-magmatic processes. The glasses of granite eutectic composition were synthesized at 1200oC and fO2 corresponding to wustite stability field by the "inside crucible" method. Corundum and graphite powders were used as lock. The experimental conditions are described in the table. A part of the glass grains was annealed on air at 500oC for 10 days.
12
Figure. Oxygen fugacity in the system Oxide (1) - Oxide (2) as a function of temperature.
The initial and annealed glass grains were let to interact with concentrated HCl. The HCl solutions were chosen, because, if antimony oxides formed on the grain surface, antimony would be lost by the solid phase on heating in air. The antimony concentrations in the solutions were measured by atomic absorption. The antimony contents in the glasses were determined by X-ray spectrometry using a Camebax-Microbeam microanalyzer (operation voltage 15 keV, probe current 30 nA) The limit of Sb detection by the line Sb L P1 is 0.05% at significance level 0.05.
No antimony minerals were found. The results of the measurements are presented in the table. The antimony concentrations in the HCl solutions that were in contact with the pre-annealed phases are several times higher than those interacting with the initial grains.
The observed difference in antimony mobility between the untreated samples synthesized at reducing conditions and the samples subjected to thermal and oxidizing action can be explained from the different redox states of antimony at fO2 of the wustite stability field and air.
Stahlberg and co-authors used the Mosbauer's method for the investigation of Sb+3/Sb+5 equilibria at different fO2 in the glass-forming melts of the composition 70% SiO2, 23% of alkali and alkali-earth metal oxides, and 0.2% of Sb2O3 (Figs. 2, 3). The oxygen fugacity in the melts was controlled by emf method. The redox equilibrium under study was presented as
2Sb+5 + 2O2- = 2Sb+3 + O2
Isomeric shifts were -12.00 mms-1 for Sb3+ and 1.34 mms-1 for Sb5+.
Thus, antimony complexes are evidently absent in silicate glasses, and, therefore, the antimony ions are highly mobile. Previously we studied the character of copper redistribution among the alumosilicate glass grains [1] and found that copper oxides formed on the glass surface when redox conditions changed. Probably, the same migration and concentration mechanism is valid for antimony as well.
Table. Antimony concetrations in the HCl solutions in contact with the granite eutectic glasses.
|
Sample no. and synthesis conditions |
Glass annealing conditions |
Glass weight, mg; |
Solution volume, ml; |
Sb concentration in the solutions being in contact with glass grains for 40 min*****; |
Loss of Sb by the glasses after interaction with HCl solutions, %; |
|
28-Sb* T-1200oC |
without annealing |
39.9 |
2 |
0.018 |
1 |
|
fO2 -FeO |
500oC, 11days fO2 (air); |
40.4 |
2 |
0.096 |
5 |
|
29-Sb** T-1200oC t-2h |
without annealing |
80.0 |
2 |
0.912 |
1 |
|
fO2 -FeO |
500oC, 10days fO2 (air); |
80.0 |
2 |
2.535 |
3 |
|
30-Sb*** |
without annealing |
80.0 |
2 |
0.260 |
1 |
|
fO2 -FeO |
500oC, 10days fO2 (air); |
80.0 |
2 |
0.820 |
3 |
*
According to local X-ray spectrometry, the Sb2O3 content in glasses was 0.13 + 0.02%**
0.3-0.5%***
1.0-0.5%****
grain size 0.2-0.5 mm*****
atomic absorption data (analysts Yu.G. Tatsii and L.N. Bannykh).References:
13
# Shchekina T.I., Gramenitskii E.N., Sval'nova V.I., and Romanenko I.M. Partition of Ta, Nb, Zr, and Hf between alumosilicate and fluid melts in the fluorine-bearing granite system.
key words [melt fluid salt ore elements]
The rare-metal granites and pegmatites enriched in Ta, Nb, Zr, and Hf are the latest products of differentiation of the normal biotite or subalkaline fluorine-bearing granites. We studied the partition of these elements between the alumosilicate and alkali-aluminofluoride (salt or fluid) melts in the system Q-Ab-Or-H2O-F at 800oC and P=1 kbar. The phase relations in this system were previously reported (Geochimiya, 1993). This system represents the composition of the fluorine-bearing magma of the latest phases of granitic massifs, which are charatcreized by various Na, K, and Li contents and Na+K+Li/Al and Si/Al ratios.
It was shown that Ta, Nb, Zr, and Hf are accumulated in the aluminosilicate melt in any part of the system (except Hf and Zr in Li-rich compositions), however, the partition constants Kpsil/salt depend on Na/K and Si/Al ratios and agpaitic coefficient. When passing from pure Na to K-Na and K-rich compositions of the system, the Ta partition constant somewhat increases, and those for Nb and Zr increase as a result of relative increase of the contents of these elements in the salt melt. The experimental studies showed these elements to favor the aluminosilicate melt, which is rich in alkalies and fluorine (up to 3 wt %). The element contents in the aluminosilicate melt can range up to 1-4 wt % depending on the starting element amounts added to the system. These data are consistent with the petrological and geochemical conceptions concerning the accumulation of these elements as a result of the crystallization differentiation, from earlier to later (including pegmatites) phases of the process. The separation of the aluminofluoride liquid enhances this effect. The relationship between the crystallization differentiation and liquation is the subject for a special investigation.
The Nb/Ta ratio decreases in the crystallization, i. e. Ta is accumulated to a greater extent. From our data, the Nb/Ta ratio (defined as a ratio of the partition constants of Nb and Ta between the aluminosilicate and the alkali-aluminofluoride melts) decreases when passing from sodium-rich to potassium-rich
compositions of the system. This fact correlates with the well-known tendency of Nb concentration in Na- and Ta-K-Na-granites.
The character of the element distribution changes markedly when lithium is added to the system: Zr and Hf become favoring the salt melt, and the Ta and Nb contents in the salt melt increase significantly, although their partition constants remain more than unity. The Li-containing part of the system is close to Li-F granites in composition. The role of the fluid melt enriched with Li and F in the accumulation of Nb and Ta is not very significant yet in the magmatic phase; separation of the fluid melt brings the same result as the crystallization does. The Nb/Ta ratio regularly decreases with increasing Li content. This fact is consistent with the natural regularities established for Li-F granites, which are, as a rule, the latest differentiation products of granitic massifs. It is of importance that Zr and especially Hf favors the fluid melt. From this fact an increase in Zr/Hf ratio during the differentiation can be explained: Zr is accumulated in the aluminosilicate melt, and Hf is concentrated in the alkali-aluminofluoride melt.
As the agpaitic coefficient of the system decreases, the Kpsil/salt values for Nb and Hf decrease, and that for Zr approaches unity, while the Ta partition tends favoring the aluminosilicate melt. The opposite behavior of Ta and Nb and less pronounced Zr and Hf has long been known in geochemistry, however, could not be explained by the element fractionation during crystallization.
We established that the immiscibility field extends to the nepheline-normative field. Transition through the feldspar barrier is accompanied by changing the partition constants: increasing for Zr and Nb and decreasing for Ta and Hf. Hence, the values of the geochemical indicators Nb/Ta and Zr/Hf for granites and nepheline syenites differ, and tantalum deposits are associated with granites, and niobium deposits with nepheline syenites.
Conclusions:
1. The accumulation of the elements under study in the fluid melt is of importance only for Zr and Hf in the Li-rich part of the system. The deposits of these elements are probably of late magmatic origin. The separation of the fluid melts enhances the accumulaton of these elements as a result of crystallization differentiation. The relationship beween two different differentiation mechanisms requires special investigation.
2. The experimental data obtained offer a simple and reasonable explanation to the well-known empiric regularities: the variations of geochemical indicators in di -
# This study was supported by the Russian Foundation for Basic Research.
14
fferent rocks and the difference in ore potentials between granites and nepheline syenites.
Maximov A.P. Influence of water on the mineral-melt equilibria: effect of dilution.
key words [melt water calculation]
Equilibrium constants of melting reactions under dry conditions are proposed to calculate the influence of water on silicate mineral-melt equilibrium temperatures. In this case the influence of water is allowed for through a decrease of the molar fractions of the melt components. The components are taken to be one-cation-base oxides. The equilibrium temperature in aqueous melts may then be derived from the equation:
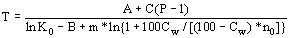 (1)
(1)
where A,B and C are constants, P is the pressure (bar), Ko is the equilibrium constant of the crystallization reaction at 1 bar, m is the number of particles forming during mineral melting; Cw is the water concentration in the melt (wt pct), W is the molecular weight of water particles, no is the total number of cations in 100 gr of the dry melt.
For the olivine-melt equilibrium the constants A,B, and C were calculated from the volumetric effect and the equation of melting of forsterite under dry conditions:
 Vfof =0.019cm3/g and T(K)=2163+0.00477P (bar).
Vfof =0.019cm3/g and T(K)=2163+0.00477P (bar).
It was obtained: A=7132.9, B=-2.3429 and C=0.0157. Analogously, for diopside: A=772.0, B=-2.5903, C=0.060. The quantity m assumes the values of 1.5 and 2 for olivine and pyroxene, respectively. To estimate the effect of dilution of the components by water on the liquidus temperature decrease, and the acceptability of this approach in principle, eq(1) together with the experimental data on water solubility were used for the calculation of melting temperatures of pure forsterite and diopside and, also, natural olivinic tholeiite and peridotite under the water-saturated conditions. The experimental temperatures fall between the theoretical temperatures calculated under the assumption of completely molecular form of the water in the melt (W=18.02) and completely hydroxyl one (W=9.01). Herewith eq(1) fairly reproduces the course of the liquidus curves in P=T and T-H2O coordinates. In order to describe adequately the experimental data the formal value, i.e. the effective water concentration (Ceff wt pct) is proposed to be used instead of Cw in eq(1). Ceff is taken to mean the concentration of water which at the given choice of w in eq(1) will ensure the same number of water particles in the melt as Cw does, with account taken of the concentrations of hydroxylic (Chydr) and molecular (Cmol) forms.
With w=9.01 we have
Cw = Chydr + Cmol (2)
Ceff =Chydr + 0.5Cmol (3)
For the olivine liquidus in olivinic tholeiite (Yoder, Tilley, 1962) from eqs (1-3), taking Tcalc=Texp , the calculated Chydr and Cmol on the Cw- Chydr , Cmol plot reproduce adequately the experimental data (see Holloway, Blank, 1994, fig.8). For the experimental data obtained by Sisson and Groove (1993 a,b) the equilibrium temperatures of olivine-melt pairs were calculated. Different models of water solubility (Burnham, 1975, 1994; AlÆmeev, Ariskin, in press Silver, Stolper, 1989) were used in the calculations. In the program for the calculation of concentrations of molecular and hydroxylic water using the model of Silver and Stolper (Holloway, Blank, 1994) a mean deviation of the calculated temperatures from the experimental ones in estimating Ceff is 15oC. This makes it possible to employ eq(1) as an olivine-melt geothermometer.
# Zharkova E.V.2, Kadik A.A.1 Megacrysts from the Shavaryn-Tsaram volcano: experimental determination of oxygen fugacity.
key words [oxygen fugacity mantle minerals]
The oxygen chemical potential is one of the most important characteristics of the thermodynamic state of the upper mantle responsible for the behaviour of volatile components and variable-valence elements in the process of differentiation of the mantle matter. The determination of fO2 (intrinsic oxygen fugacity) values inherent in deep redox reactions is a problem solvable either by thermodynamic analysis of mineral equilibria or by direct experimental determination of fO2 of minerals using solid-state electrolytic cells.
In this work we report the determinations of oxygen fugacity of clinopyroxene (Cpx), sanidine (Sa), garnet (Cr) and pyrope (Py)megacrysts from the Shavaryn-Tsaram volcano (Mongolia). The measurements were performed within 800-1100oC at 1 atm on a high-temperature apparatus comprising two solid electrolytes on the base of zirconium oxide stabilized with yttrium oxide. The use of two electrochemical cells enables the determination of the fO2 of minerals having a small buffering capacity with respect to oxygen. The measured values of the oxy -
# The work has been supported by the Russian Basic Re-search Foundation (grant N 96-05-64954).
15
gen fugacity of megacrysts obey the linear dependence of the type: log fO2 =A-B/T, where T is the temperature in the Kelvin scale.
The results of the experiments are listed in the Table:
|
Sample |
A |
B |
r |
n |
|
Cpx |
20.672 |
41849.1 |
0.995 |
10 |
|
Sa |
17.516 |
37903.7 |
0.998 |
8 |
|
Gr |
16.491 |
36482.4 |
0.997 |
9 |
|
Py |
13.897 |
34403.5 |
0.994 |
9 |
where n is the correlation coefficient, n is the number of the experimental points. The conducted runs have shown that the oxygen fugacity of Cpx, Sa, and Gr are practically identical and are located 1-1.5 order higher than the buffer equilibrium of WM whereas the oxygen fugacity characteristic of Py megacryst is close to the WM buffer equilibrium. The comparison of the obtained fO2 for the megacrysts with the oxygen fugacity data for spinel lherzolite xenolites from the Shavaryn-Tsaram volcano (Geokhim., 1988, N.6, pp.783-793) suggests that their formation is characterized by the oxygen fugacity values close to the WM buffer equilibrium.
# Epel'baum M.B. Correlation of water forms in a melt giving the information on the interaction melt-water.
key words [water melt interaction albite]
New data have appeared for more than 10 years and the results are actively discussed on the estimates of water forms in natural and model magmatic melts. The stereotypes of presentation of experimental data have been formed for this time. The present work shows that the data, if considered in other coordinates, may lead to new conclusions and bring corrections to the idea of water-melt interaction
The 'conventional' picture (Stolper, 1982) involves the dependences of contents of molecular water and water in a hydroxyl form , i.e. of water interacted on the summary water dissolved in a melt. Such pictures prompt the conclusion that there exist two regions differeing in the character of dissolution of water in both forms. Herewith, on the second region, OH groups already do not enter the melt and the tangent of the line slope of the molecular water content equals unity. Hence, one can suppose that all the dissolving water enters the melt in the molecular form. In reality it is not so. On the second region the melt goes on depolymerizing with addition of hydroxyl water. This follows from considering the similar dependence in case all the water forms and total water are represented as a number of moles accounted for a mole of the melt. The water-to-melt unit ratio makes it possible to consider in the relation analysis both the water forms and silicate melt. So, such a consideration is more informative. In particular, a continuous growth of a number of hydroxyls, however less intensive, is observed in the second region too.
New information can be obtained if one instead of the concentration curves of separate water forms considers the value of molecular water- hydroxyl water ratio. We have already come to the conclusion that in all the composition range studied, dissolving H2O enter the melt in both forms. This is to say that water forms are in equilibrium. Many authors calculate the reaction constant to write it in any form. However, the possibility of a rough description of water dissolution as two step-by-step reactions remains attractive: a) dissolution of water in the form of H2O and than b) in the molecular form. When considering the dependence of the molecular water- water in the OH form ratio on the summary water (in wt%) aloows to visually estimate such a possibility. We have considered the data of Silver, Stolper, 1999 for the water bearing albite melt. We have plotted them on the web of hypothetic lines where the first reaction terminates at 1; 1.5; 2; 3; 5 wt%. It occured that the suggestion about the two stage dissolution of water may be used as a very rough approximation. Experimental curve actually has two rectilinear regions with a smooth transition between them. So, widely adopted approach to water dissolution in two forms as an equilibrium of reaction products in the homogeneous phase is virtually more consistent with actual data. It is interesting that the data for different melts presented on the same diagram illustrate their relative chemical interaction with water. These data are possible to be used as an additional criterion of the estimation of water acidity with respect to the melt.
When analysing the diagrams of H2O(mol): H2O(OH) as a function of H2O(tot)m one can make some assumptions on the character of interaction, quantity, and, perhaps, the type of the particles formed. For this purpose we compared the experemental curves of the dependence of the water forms ratio in an albite melt against its total content (in mole parts) with the calculated isolines at the constant values of the constant of equilibrium reaction. The reactions used are as follows:
NaAlSi3O8 + H2O = NaHSiO3 + AlH(SiO3)2 (1)
2NaAlSi3O8 + H2O = 2Al(H9SiO3)2 + Na2Si2O5 (2)
NaAlSi3O8 + H2O = NaAlSi3O7(OH)2 (3)
The most common in literature reaction (O + H2O = 2OH) is omitted here because it is a most
# The work was supported by the Russian Basic Research Foundation (grant N 95-05-14572)
16
general record and neither differentiate the oxygen types in albite nor bears no information on the chemical composition of the particles formed. When it is assumed that in water bearing glasses the equilibrium constant remains stable or at least close to stable, then the comparison of isolines of these constants for the three reactions mentioned with the experimental results may be an argument for the choice of the reaction. It turned out that experimental points are most correspondent to the reaction 2.
The proposed analysis of the H2Omol.-to-H2O hydrat. ratio bears additional information for the elucidation of the mechanism of water interaction with alumosilicate melts.
References:
# Lebedev E.B. and Kadik A.A. Experimental study of anorthosite formation with the high-temperature centrifuge.
key words [anorthosite melt differentiation]"
The mechanisms determining the evolution of the partial melting zones in the earth's crust and mantle are presently of great interest for researchers. These mechanisms can be investigated experimentally using centrifuges. Liquid heterogeneous mixtures are efficiently separated in centrifugal fields, where the 3000-fold strengthening of the earth's gravity field is attained, and the initial melting degree ranges up to 20-60 vol %.
The centrifugal experiments with gabbro and basalt samples at 1180-1190oC showed that the samples undergo differentiation to yield three distinct zones, with the melt accumulating in one of the zones:
1. The lower fraction of the sample represented by the crystalline residue with various degree of phase differentiation.
2. The middle fraction of the sample where the melt accumulates.
3. The upper fraction of the sample represented by emersed plagioclase crystals and some quantity of captured magmatic liquid.
The results of the centrifugal experiments fit the conceptions about the conditions of matter differentiation on the earth and the Moon in the course of large-scale melting. We mean the assumption as to the three main zones formed: (1) the anorthosite layer with some quantity of captured magmatic liquid; (2) the melt layer, and (3) the thick layer composed of residual high-melting ferromagnesian crystals variously differentiated.
This conception of the partial melting zone evolution correlates well with the theoretical calculations and modeling performed by M.Ya. Frenkel'.
In centrifugal field, all the samples exhibited the differentiation that appeared as melt accumulation and floating up plagioclase crystals forming the "anorthosite" layer at 1180-1190oC. Plagioclase and therefore anorthosites have lower density than basalt melt. The phase crystallization order is of minor importance in dry systems, since all the phases crystallize simultaneously for a long time. However, the oxygen fugacity variation may cause the change in phase crystallization order.
The performed experiments showed that the separation of the silicate liquid from crystals is determined by the multiphase matter flow which may cause the layered phase distribution. The melt yields a separate zone above which the anorthosite layer composed of the emersed plagioclase crystals and some quantity of captured magmatic liquid is formed.
## Sushchevskaya N.M. and Tsekhonya T.I. . Petrochemical types of ocean-ridge tholeiites and evaluation of their Formation conditions from computer modelling data.
key words [tholeiite mid-ocean computer modelling]
We studied mid-ocean-ridge (MOR) tholeiites of Central, Equatorial, South Atlantic, Indian Ocean, and East Pacific Rise (EPR) at lat. 23o north to 25o south. Comparison of tholeiitic magmatism of MOR segments differing in geodynamic setting made it possible to distinguish tholeiites with different conditions of the original magma generation in the dry lherzolite mantle beneath the mid-ocean ridges.
The conditions of tholeiite crystallization were evaluated by comparison of the parameters of the original melt fractionation obtained from the KOMAGMAT model [1] with petrochemical data of natural glasses. The original melt composition is uncertain in some cases, and choosing the composition for the modeling is a serious problem. The composi -
# The present study employed the original equipment pat-ented at. no 95105802/02 (Russian Federation) and was supported by the Russian Basic Research Foundation, project no. 95-05-15292.
## This study was supported by the Russian Basic Research Foundation, projects no. 96-05-65569.
17
tions of phenocrysts were used for the simulation of the original magma fractionation.
The shallow-depth TOR-2 tholeiites are most abundant among the tholeiite types studied [2]. The original melt composition for TOR-2 was determined from the actual compositions of homogenous inclusions in the most magnesian olivines from the tholeiites [3]. The model fractionation paths obtained for this original melt describe well the position of composition fields of many natural glasses of this type. The estimated crystallization parameters for TOR-2 tholeiites from slow-spreading (Indian-Atlantic Province) and fast-spreading (EPR) ridges differ respectively as: 3-4 bar, 1270-1170oC, maximum fractionation degree 50% and 0.001-2 kbar, 1270-1150oC, maximum fractionation degree 70%.
The Na-tholeiite type, which was distinguished by Klein and Langmuir [4] on the basis of Na content in glasses reduced to 8% MgO (Na8>3), is characterized by wide dispersion of Si8 (50-53) and Fe8 (7-10). According to the present models, the melts enriched significantly in Na and Si and depleted in Fe could originate as a result of complex polybaric (in the pressure range 15-4 kbar) critical melting of the original mantle, with a part of melt constantly remaining in the mantle matrix (about 1 vol %) [5]. The estimated parameters of Na-melt crystallization for the Romanche Fracture Zone are 4 kbar, 1260-1170oC, and crystallinity no more than 50%.
A specific type of tholeiitic melt enriched in Si and depleted in Ti and Na, established only in the Indian Ocean, is mainly associated with early phases of the ocean formation. Examination of different original melts within different models [5, 6] showed the melts described by Niu and Batiza [6] to yield the best fit. The melts with low Na and Ti contents and high CaO/Al2O3 ratio can be obtained by the modeling of fractionation polybaric (20-4 kbar) melting. According to this model, Si-tholeiites crystallized at low pressures (0-2 kbar) and within the temperature range 1210-1150oC. Formation of siliceous tholeiites probably resulted from profound melting of the depleted mantle. Occurrence of these rocks largely in ancient regions of the ocean indicates the more high-temperature conditions of the early stages.
The petrochemical parameters of fractionation of the melt that formed at low degrees of melting (6%) and slight pressure decrease (20-19 kbar) [6] mirror the main characteristics of the alkaline glasses found in the Equatorial Atlantic. From calculated data, crystallization of alkaline melts proceeded at higher pressures (6-8 kbar) in comparison with most of tholeiitic melts. The fractionation degree of such melts was no more than 40%.
Thus, in spite of different formation conditions of the original melts, the conditions of fractionation of tholeiitic melts beneath most recent slow-spreading ridges are similar. These melts crystallize at pressures at least 3-4 kbar, while the melt fractionation in the fast-spreading settings occurs at subsurface conditions, and these melts are more differentiated.
References:
18