Infrared spectra of water in volcanic glasses#
Y. Yanev1, N. Zotov2
1Geological Institute, Bulgarian Academy of Sciences, Sofia 1113
2
Laboratory of Mineralogy and Crystallography,
Bulgarian Academy of Sciences, Sofia 1000, Bulgaria
ABSTRACT
Infrared and near-infrared spectra have been taken on 9 perlites (2-6.7wt% H2O) from the Eastern Rhodopes, Bulgaria and 3 obsidians (0.24-0.77wt% H2O) from Sardinia, Island, and Mexico.
Comparing the spectra of the perlites before and after heating at different temperatures (275-1200oC) with the spectra of the obsidians the shoulder at 3430-3490 cm-1 has been attributed to OH stretching vibration of hydroxyl groups connected with the glassy structure whereas the absorption maximum at about 3590-3595 cm-1 and the shoulder near 3220- 3240 cm-1 to OH strretching bands of two types of molecular water.
The position of the 3600 cm-1 band decreases with increasing albite content of the glasses. The OH absorbance at 4545 cm-1 in the NIR spectra shifts to higher frequences after heating and persists even at 1200oC. The position of the absorption bands permits to determine the dominant average bond distance R (O...O) of the hydroxyl groups in the investigated rhyolitic volcanic glasses at about 2.9 Å, and those of the two types of molecular water 2.8Å (ice type H-bond) and 3.1 Å. The distances O-H of all water species are 0.94-0.97 Å.
Introduction
A huge amount of literature is devoted to the IR spectra of water in model and volcanic glasses. Only a brief review of the most important results concerning the IR (3000-4000 cm-1 range) and the near-infrared, NIR (4300-8300 cm-1 range) of synthetic and volcanic glasses, which are the subject of the present paper, will be given here. Historically, Ellis and Lyon (1936) first suggested that the absorption maximum at about 3600 cm-1 in SiO2 glass is due to the presence of water in the glass. Later this was confirmed by Harrison (1947). Glaze (1955) observed two absorption maxima in binary, ternary, and commercial glasses at about 3600 and 3400 cm-1, which he attributed to molecular water.
However, Moore and McMillan (1956) and Adams (1961) attributed the peak at 3400 cm-1 to OH groups, connected with the glassy matrix. Ernberger (1977) has published IR spectrum of the Si-Al-B-Na water-bearing glass with asymmetric maximum about 3600 cm-1, that he attributed to the molecular water (eventhough in the text he writes about a strong absorption of the molecular water at 3410 cm-1).
The first authors to investigate volcanic water-containing glasses (perlite with 3.34 wt% H2O) in the 3000-3600 cm-1 range were Keller and Pickett (1954). they assumed the presence of monomeric OH groups and hydrogen bonded molecular water and proposed a structural model of the hydrogen bonds in the glass. Nasedkin (1963) and Nasedkin and Panesh (1967) published a series of IR spectra of obsidians and perlites. According to these authors only OH groups are present in the obsidians, while both OH groups and molecular water occur in the perlites. Epel'baum et al. (1975) on the base of IR spectra of the perlites and obsidians examined the influence of water on the glass structure on the coordination of aluminium in particular. Zibrova (1981) performed IR studies of heat- treated volcanic glasses at different temperatures. On the basis of the temperature behaviour of the different absorption bands in the 3200-3600 cm-1 range she attributed the 3590-3620 cm-1 band to vibrations of OH groups bonded to metal cations, and the absorption bands at about 3400-3500 cm-1 and 3000-3200 cm-1 to molecular water species. The investigation of volcanic glasses in the NIR range begins with the paper of Cohen (1958) in which the spectrum of the Jamez Mt perlite (4 wt% H2O) is published.
# The paper have been prepared under the project N 528 of the National Science Fund of Ministry of Education, Science, and Technology (Bulgaria).
1
The fundamental works of Scholze (1959, 1966) confirmed the presence of hydroxyl groups and H2O species in Na-silica glasses on the basis of separation of the corresponding absorption bands (at about 4500 and 5200 cm-1) in the NIR spectrum. Orlova (1962) first investigated hydrous glasses with albite composition, i.e. glasses with composition close to that of volcanic glasses, and established the same absorption bands as Scholze (1959, 1966). In his second paper Scholze (1966) reinterpreted the IR data of Cohen (1958) for the Jemez Mt perlite and showed that both molecular water and small amount of hydroxyl groups coexist in it. A new significant step in understanding the structural role of water in silicate glasses was laid down by the fundamental papers of Bartholomew et al. (1980) and Stolper (1982) for volcanic glasses. In the first study the spectrum of the glass with the low water content (0.021 % H2O) has the absorption maxima only at 3484 and 4505 cm-1 attributed to the OH groups; the spectrum of the glasses with high water content (7.9 % H2O) has a wide asymmetric maximum between 3600 and 3200 cm-1 ( the both glasses are of Al-K-Zn-Na-Si composition with 77% SiO2). Using the absorption bands in the NIR range they determined the quantity of OH and molecular H2O, so the ratio of H2O/OH, which is constant at given temperature in the melt. It was shown that the hydroxyl groups are the dominant H-bearing species below 4.5 wt% total water, molecular water prevails at total water grater than 4.5 wt%. These investigations were continued in the papers of Newman et al. (1986), Stolper (1989) and Silver et al. (1990). In particular, it was demonstrated that the glass composition and the synthesis temperature also have an effect on the speciation of OH bonds in the glasses.
Raman spectra of water-bearing glasses (with 4 and 6.6 wt% H2O) of albite and Ab37O63 (components in mol%) compositions also reproduce the broad and asymmetric absorption band about 3600 cm-1 with a shoulder 3250 cm-1 (McMillan et al., 1983, 1993).
The present IR studies are the continuation of the spectroscopic investigation of water-containing Paleogene glasses with rhyolite composition from Eastern Phodopes (Bulgaria), published by Dimitrov et al. (1984). The aim of the present paper is comparing the IR and NIR spectra of glasses with low-water content (obsidians) and high-water content (perlites) before and after heating:
1) to attribute the different bands in the large maximum between 3000-4000 cm-1 to different H-bonds in volcanic glasses (H2O and/or OH) and thus
2) to determine, on the basis of comprehensive works of Nakamoto et al. (1955) and Novak (1974), the most probable average distances R(O...O) and O-H in different types of H-bonds in the glasses. That is a supplementary point of our X-ray diffraction (Zotov et al., 1989, 1992) and neutron-diffraction (Zotov et al., 1995) studies on the structure of water-bearing rhyolite glasses.
Experimental
The investigated samples represent perlites (glasses with H2O> 1 wt%) of Late Paleogene age collected by us from the Eastern Rhodopes, Bulgaria and for comparison, the obsidians (glasses with H2O< 1 wt%) of Late Neogene age from Monte Arci, Sardinia (Italy), Western Siera Madre (Mexico) and Quaternary volcano Hraftinuskur (Island). Their chemical composition is present in Table 1 and their normative mineralogical composition (according to CIPW method) in Table 2. The total water contents were determined at loss-on-ignition at 900oC.
Table 1. Location and composition of the investigated volcanic glasses.
| Symbols
| Sard
| Isl
| Ort
| Tat
| Gol
| Sil
| Dam
| Svet
| Sar
| Loz
| Bor
|
| Location
| Sardi-
nia
| Iceland
| Eastern Rhodopes
|
| Volcano or area
| Monte Arci
| Hraftin-
nuskur volcano
| Ustren area
| Tatare-
vo area
| Studen Klade-
netz v.
| Silen
volca-
no
| Damba-
lak vo-
lcano
| Studen
Klade-
netz v.
| Boro-
vitza
volca-
no
| Lozen
volca-
no
| Boro-
vitza
calde-
ra
|
| SiO2
| 75.83
| 74.86
| 73.88
| 69.88
| 70.14
| 73.49
| 66.94
| 70.35
| 70.28
| 70.02
| 69.60
|
| Al2O3
| 13.18
| 13.67
| 11.56
| 14.31
| 13.49
| 12.29
| 14.20
| 14.30
| 11.68
| 12.10
| 12.17
|
| Fe2O3
| 1.60
| 2.93
| 0.88
| 2.58
| 1.65
| 1.00
| 1.68
| 1.93
| 1.72
| 0.88
| 0.96
|
| MgO
| 0.18
| 0.22
| 0.30
| 0.28
| 0.23
| 0.24
| 0.32
| 0.20
| 0.25
| 0.22
| 0.20
|
| CaO
| 0.65
| 0.21
| 0.66
| 1.92
| 0.60
| 1.11
| 0.96
| 1.52
| 1.58
| 0.81
| 1.95
|
| Na2O
| 2.42
| 2.99
| 2.79
| 3.26
| 2.86
| 2.40
| 2.43
| 2.70
| 2.69
| 3.09
| 3.43
|
| K2O
| 5.06
| 4.76
| 4.87
| 4.87
| 6.62
| 4.56
| 6.61
| 4.20
| 3.48
| 3.78
| 2.28
|
| H2O+
| 0.24
| 0.28
| 2.08
| 2.18
| 3.60
| 3.72
| 4.20
| 4.71
| 5.21
| 6.04
| 6.70
|
2
Table 2. CIPW norms of the investigated perlites from Eastern Rhodopes.
Glasses
| Q
| Or
| Ab
|
Ort
| 41.63
| 32.07
| 26.30
|
Tat
| 31.87
| 34.79
| 33.34
|
Gol
| 28.99
| 43.88
| 27.14
|
Sil
| 45.44
| 31.10
| 23.44
|
Dam
| 29.02
| 46.51
| 24.48
|
Svet
| 41.75
| 30.34
| 27.92
|
Sar
| 46.05
| 25.61
| 28.34
|
Loz
| 42.33
| 26.57
| 31.10
|
Bor
| 46.16
| 17.07
| 37.77
|
The perlites are composed of the glassy matrix with feldspar microlites and a small amount (<10%) of phenocrysts of quartz, sanidine, plagioclase, and biotite, as well as accessory minerals: magnetite, apatite, zircon, and titanite. The petrologic aspects of the Eastern Rhodopes perlites are summarized by Yanev (1987).
The IR spectroscopic measurements carried out in the Laboratoire de Mineralogie & Cristallographie, Universite de Paris 6 et 7 at room temperature are as follows:
a) on powder samples in dry atmosphere using FTIR Nicolet 5DX spectometer and KBr pellets in the 2700-4300 cm-1 range;
b) on powder samples using Carry 2300 spectometer and reflection geometry in the 4300-8300 cm-1 range.
The powder samples are ground in agate mortar after magnetic and electromagnetic separation of the glassy matrix from the phenocrysts and magnetite.
In order to exclude a possible influence of surface absorbed water (Newman et al., 1986) and to obtain more precise values for the position of the absorption maxima (Ziborova, 1981) doubly-polished plates (thickness 30  n) of the perlite samples were also used. They were mounted with haraldite on glass substrates. The measurements were carried out in transmission geometry using selected areas of pure volcanic glass on the FTIR Nicolet 5DX spectrometer. The spectra were recorded only between 4000 and 3420 cm-1 because at lower frequences the spectrum is masked by the absorption bands of the haraldite. The doubly-polished plates were measured in the NIR range also. The spectra obtained do not differ from the corresponding powder spectra.
n) of the perlite samples were also used. They were mounted with haraldite on glass substrates. The measurements were carried out in transmission geometry using selected areas of pure volcanic glass on the FTIR Nicolet 5DX spectrometer. The spectra were recorded only between 4000 and 3420 cm-1 because at lower frequences the spectrum is masked by the absorption bands of the haraldite. The doubly-polished plates were measured in the NIR range also. The spectra obtained do not differ from the corresponding powder spectra.
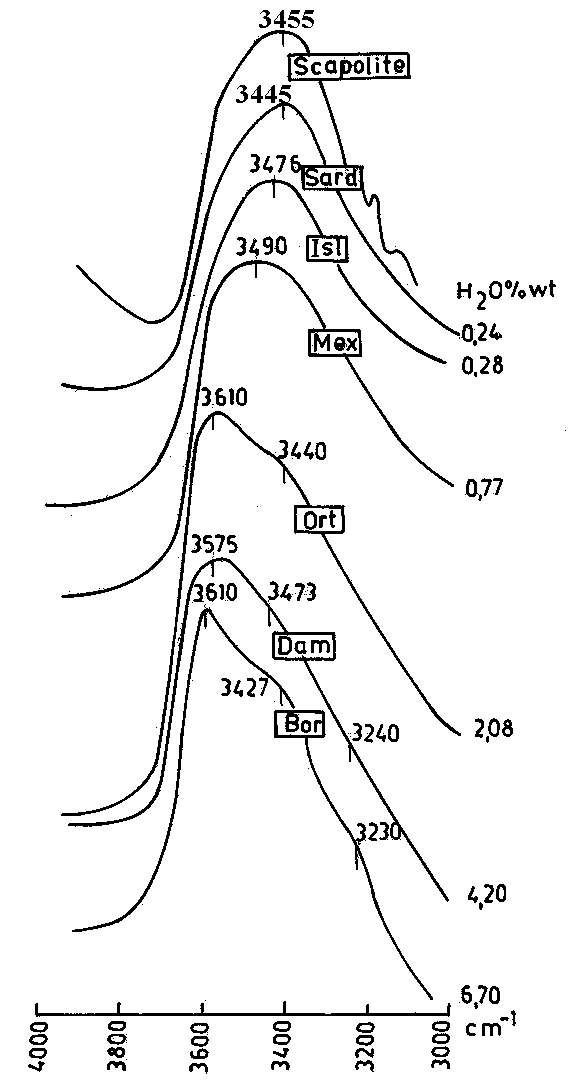
Fig.1. Typical IR spectra of the investigated glasses (obsidians and perlites) in the 3000-4000 cm-1 range. The spectrum of scapolite is given for comparison.
Several perlite samples were heated in the muffle furnace in air at 375, 475, 700, 800, 1000, and 1200oC (accuracy +11150oC above 500oC) for 2h. The corresponding temperature in the furnace was reached before placing the samples in the furnace.
Results
IR spectra (3000-4000 cm-1 range)
Typical FTIR spectra of the investigated obsidian and perlite samples are given in Fig.1. A broad strongly asymmetric absorption band is observed for all the samples which is attributed to the different types of fundamental OH stretching vibrations. It is believed that it is impossible to separate these different types in this range. While Stolper (1982) and Newman et al. (1986) practically do not observe any difference in the shape and asymmetry of the absorption band for glasses with different water content, however the samples, investigated in the present study (but with higher water content) exhibit substantial and symmetric vibrations. They concern the position, the intensity as well as the asymmetry of the absorption band. These variations cannot be attributed to water absorbed on the surface (if any) or the powder form of the samples since they are observed in the spectra of the doubly-polished plates also.
3
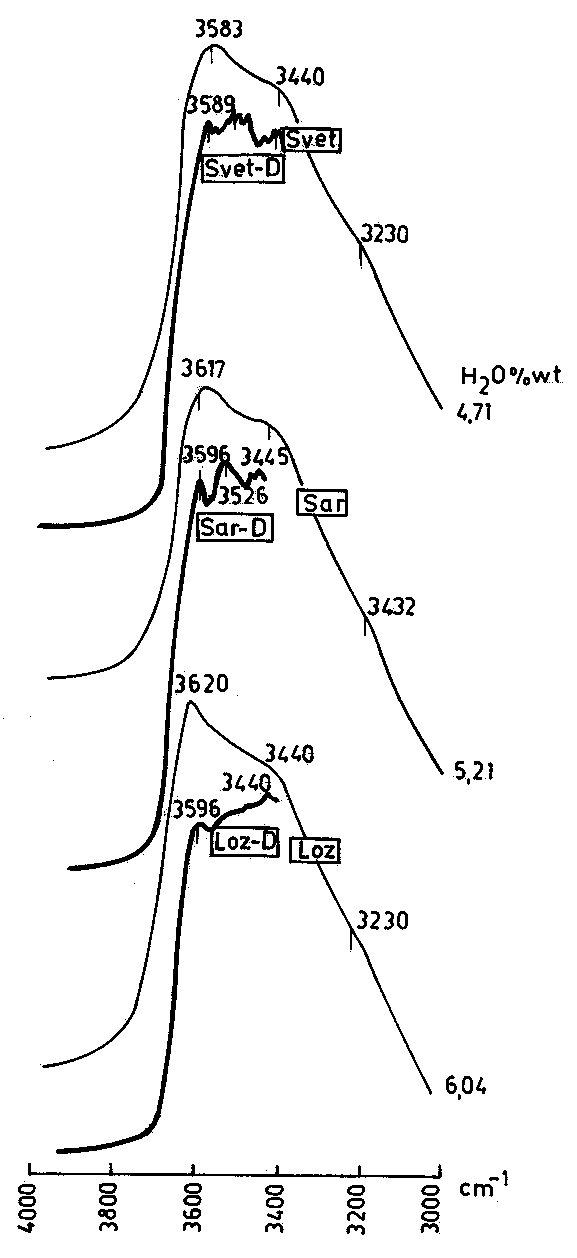
Fig.2. IR spectra of perlites before (full lines) and after heating (dashed lines).
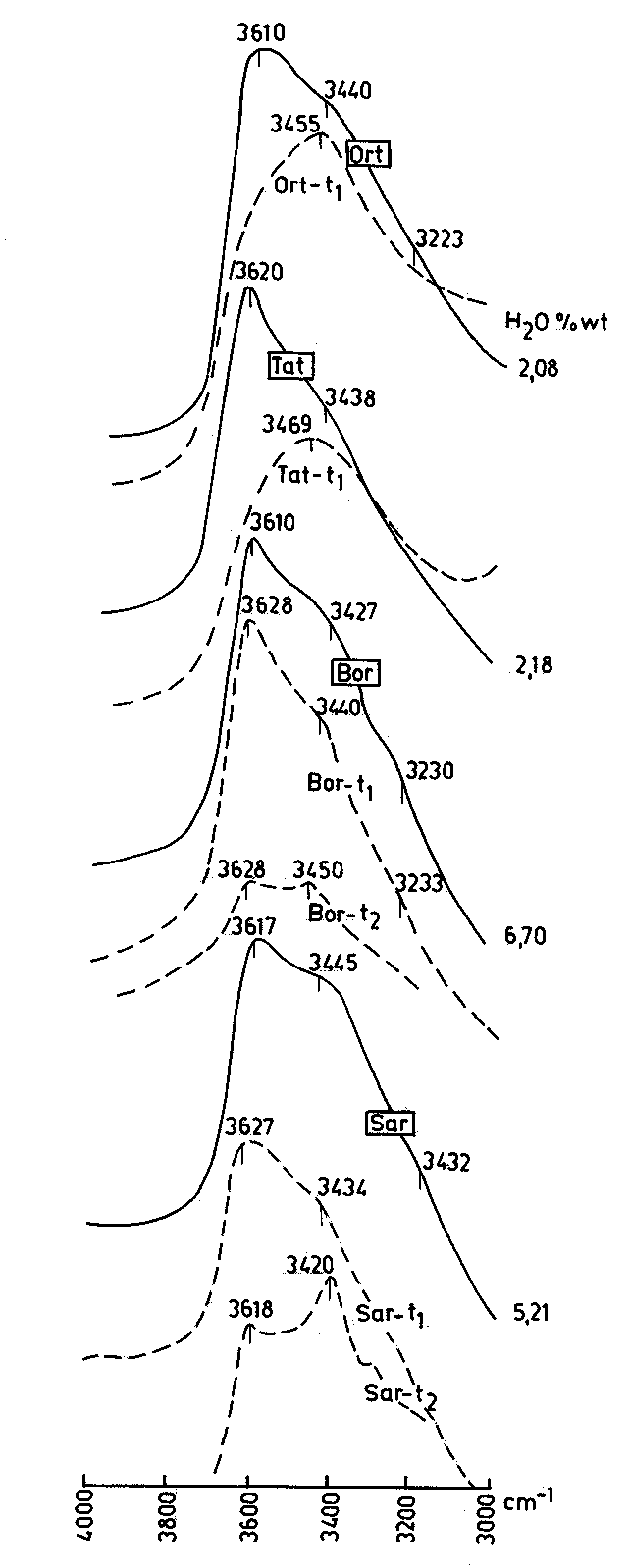
Fig.3. Comparison of the IR spectra of perlites as KBr pellets (thin lines) as doubly-polished plates (thick lines).
4
Table 3. Absorbtion maxima in the IR (300-400 cm-1) spectra of the investigated glasses.
| Glasses
| Absorbtion maxima
|
| Sard-1
| shoulder
| -
| 3445
| -
|
| Isl
| shoulder
| -
| 3476
| -
|
| Mex
| -
| -
| 3490
| -
|
| Ort
| 3610
| -
| 3440
| 3223
|
| Ort-t375
| shoulder
| -
| 3455
| -
|
| Tat
| 3620
| -
| 3438
| shoulder
|
| Tat-t375
| shoulder
| -
| 3469
| -
|
| Gol
| 3588
| -
| 3450
| 3233
|
| Sil
| 3624
| -
| 3448
| 3223
|
| Sil-D
| 3589
| 3511
| 3440
| -
|
| Dam
| 3575
| -
| 3473
| 3240
|
| Svet
| 3583
| -
| 3440
| 3230
|
| Svet-D
| 3589
| 3533
| 3448
| -
|
| Sar
| 3617
| -
| 3432
| -
|
| Sar- t375
| 3627
| -
| 3445
| -
|
| Sar- t500
| 3618
| -
| 3420
| -
|
| Sar-D
| 3596
| 3526
| 3447
| -
|
| Loz
| 3620
| -
| 3440
| 3230
|
| Loz- t375
| 3624
| -
| 3454
| 3233
|
| Loz- t500
| -
| -
| 3412
| -
|
| Loz- D
| 3596
| -
| 3440
| -
|
| Bor
| 3610
| -
| 3427
| 3230
|
| Bor- t375
| 3628
| -
| 3450
| 3233
|
| Bor- t500
| 3628
| -
| 3450
| -
|
Note: the samples denoted with D are doubly-polished plates and with t are heated at corresponding temperature.
The positions of the observed absorption bands are given in Table 3. The maximum in the obsidians is at 3455-3490 cm-1 (Fig.1), while in the perlites the strongest absorbance is at 3600 cm-1 (3590-3595 cm-1 in the spectra of the doubly-polished plates - Fig.2), accompanied by shoulders at 3430-3470 cm-1 and 3220-3240 cm-1. The latter is most pronounced in the spectra of the perlites with high water content (samples Bor and Loz) and probably it provokes the asymmetry of the large absorption band, observed in other authors' spectra (e.g. Stolper, 1982).
The spectra of the perlites change substantially after heating (Fig.3). In the perlites with low water content (samples Ort and Tat) after heating at 374oC the maximum about 3600 cm-1 disappears and this one near 3450 cm-1 becomes principle and more symmetric. In the perlites with high water content that occurs after heating at 500oC (sample Sar), and in some cases (sample Bor) after heating at higher temperature. Similar changes of the IR spectra of a perlite sample heated up to 500oC were observed recently by Semienov and Ziborova (in:Genesis of perlite, 1992).
NIR spectra (4300-8300 cm-1 range)
Fig.4 shows the NIR spectra for the glass samples examined in this study. As in all the previously published spectra, a strong absorption maximum is observed between 4515 and 4565 cm-1 in all the samples. In the spectra of the perlites the second absorption maximum is observed between 5250 and 5320 cm-1 with additional weak absorption maxima in the 5050-5155 cm-1 range. Both maxima, especially that at 5350-5320 cm-1 are asymmetric.
The absorption band at about 4545 cm-1 is attributed to combination stretching + bending mode of Si-OH and probably Al-OH vibrations (Scholze 1959, 1966), while the maximum at about 5260 cm-1 is produced by the combination mode of O-H stretching and HOH bending vibrations of the molecular water groups.
The behaviour of the two main absorption bands in the NIR range after heating is different (Fig.5). The 4545 cm-1 band decreases in intensity and shifts to a little higher frequency (especially for the perlites with low water content - samples Ort and Tat) with increasing temperature, but it is still observed up to 1200oC (including in the industrially expanded perlite).
The second absorption maximum about 5260 cm-1, as well as the additional weak maxima, decreases systematically in intensity with increasing temperature. In the perlites with low water content (Ort, Tat) it disappears after heating at 500oC, but in the perlites with high water content it is slightly visible even after heating at 800-900oC (samples Loz, Sil, Sar, and Bor). Therefore, increasing temperature of heat treatment results in higher hydroxyl to molecular water ratios as is also observed by Stolper (1989).
Discussion
Comparing the spectra of the perlites before and after heating it can be seen that in the NIR spectra the maximum at about 5260 cm-1 due to the molecular water decreases, and practically disappears after heating to 800-900oC. The same behaviour has the absorption maximum at about 3600 cm-1. Therefore, it can be assumed that the 3600 cm-1 band is mainly due to OH-stretching vibrations of molecular water species, weakly bounded to the glassy structure. Such an absorption maximum is observrd also in the spectrum of Ernstberger (1977) with 7.1 wt% H2O in the investigated glass and in the Raman spectra of albite glasses with 4.5 and 6.6 wt% H2O (McMillan et al., 1993). According to the diagrams of Bartholomew et al. (1980) and Stolper (1982), the molecular water species should be dominating. Of course, OH-stretching vibrations of hydroxyl groups also contribute to the intensity of this band (Newman et al., 1986).
The weak absorption band between 5050 and 5155cm-1 has the same behaviour and probably it is also due to molecular water species, connected with relatively stronger hydrogen bonds.
Using the data for the position of the absorption band of molecular water at about 3600 cm-1 in the investigated rhyolite perlites (composition Q41.8-46 Or25.6-30.3 Ab23.5-31.1 - see Table 2) as well as in albite (McMillan et al., 1983) and quartz-albite (Q63 Ab37 - McMillan et al., 1993) glasses it can be seen that the position of the 3600 cm-1 absorption maximum decreases with increasing "albite" contents of the glasses (Fig.6). Similar conclusion was made by Scholtz (1966) concerning the role of Na as a modifier cation in the silica glasses.
5
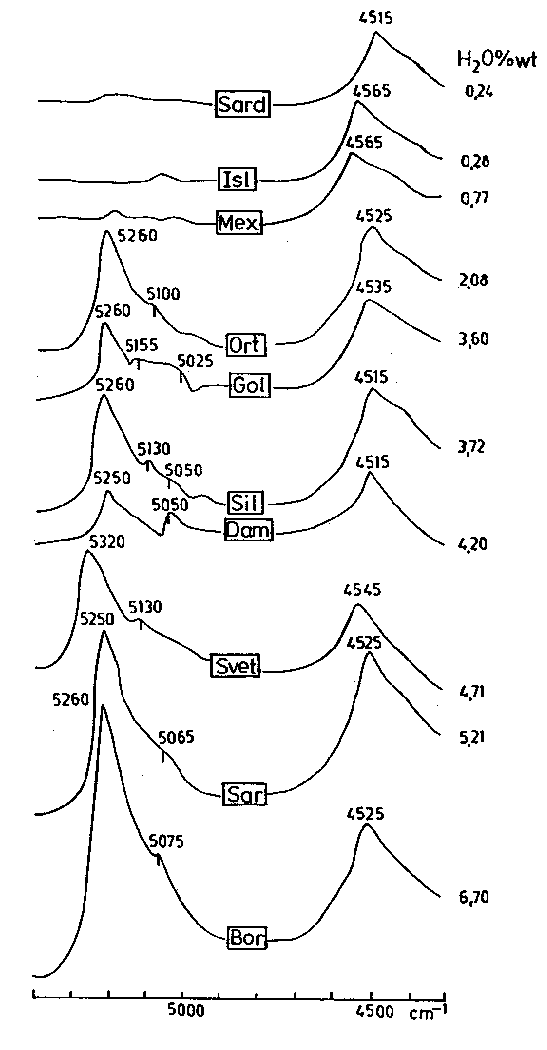
Fig.4. Typical NIR spectra of the investigated glasses (obsidians and perlites).
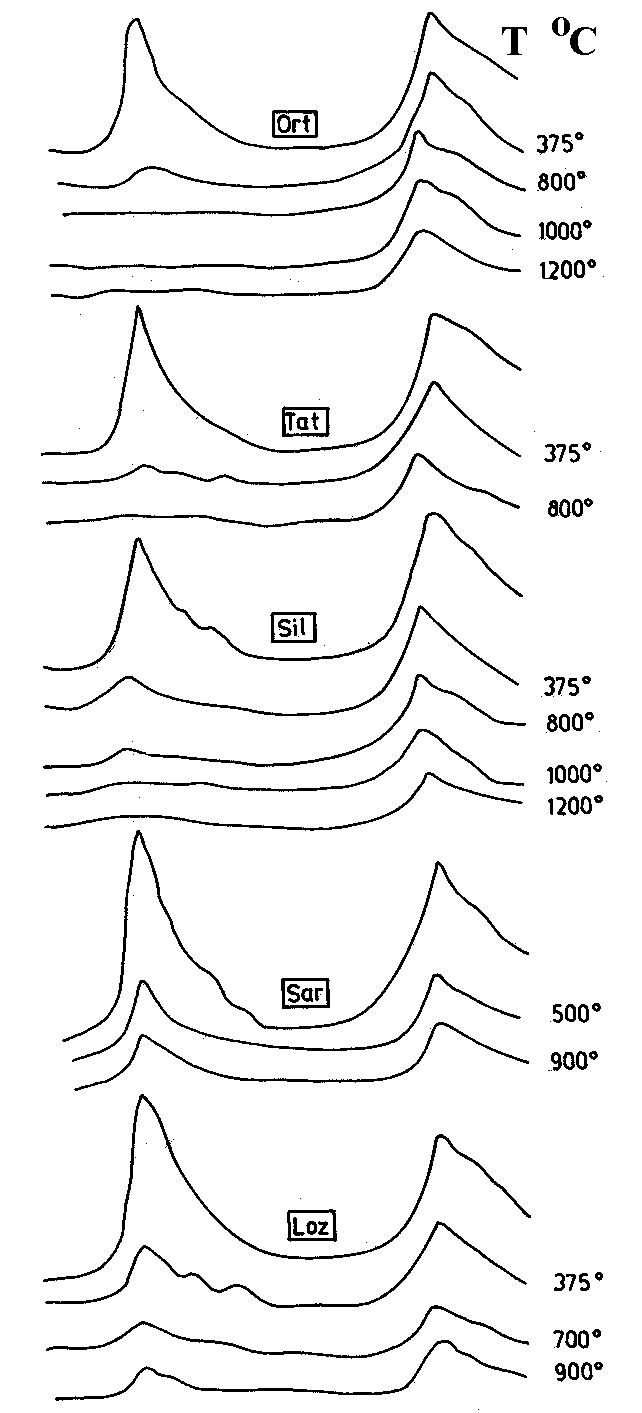
Fig.5. NIR spectra of perlites before (full lines) and after heating (dashed lines). The temperatures of heating are given above each curve.
6
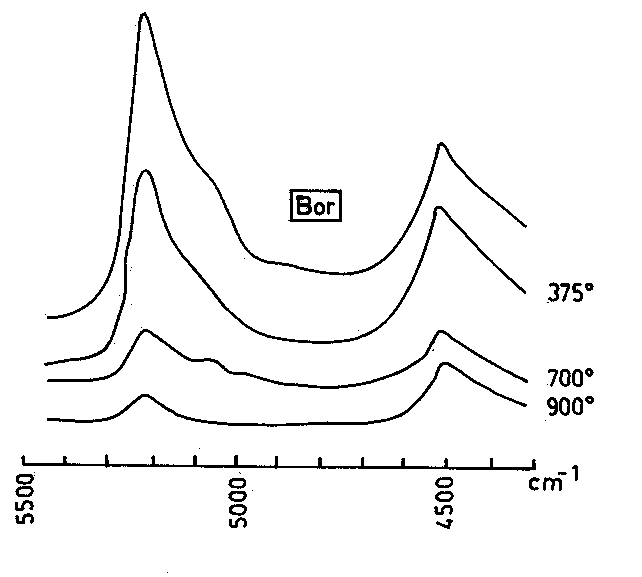
Fig.6. Dependence of the OH-stretching frequency of the molecular water at 3600 cm-1 on the "albite" content of glasses: (1) this study (doubly-polished plates); (2), (3) Raman spectra according to McMillan et al. (1993).
Table 4. Absorbtion maxima in the NIR (4500-5500 cm-1) spectra of the investigated glasses.
| Glasses
| Absorbtion maxima
|
| Sard
| -
| -
| -
|
| 4515
|
| Isl
| -
| -
| -
|
| 4565
|
| Mex
| -
| -
| -
|
| 4565
|
| Ort
| 5260
| 5100
| -
|
| 4525
|
| Ort-t375
| 5220
| -
| -
|
| 4525
|
| Ort-t800
| -
| -
| -
|
| 4565
|
| Ort-t1000
| -
| -
| -
|
| 4565
|
| Ort-t1200
| -
| -
| -
|
| 4565
|
| Tat
| 5250
| shoulder
| 4525
|
| Tat-t375
| 5235
| -
|
| 5000
| 4525
|
| Tat-t800
| -
| -
| -
|
| 4555
|
| Gol
| 5260
| 5155
|
| 5025
| 4535
|
| Sil
| 5260
| 5130
| 5050
|
| 4515
|
| Sil-t375
| 5290
| -
| -
|
| 4565
|
| Sil-t800
| 5260
|
|
|
| 4565
|
| Sil-t1000
| -
| -
| -
|
| 4565
|
| Sil-t1200
| -
| -
| -
|
| 4565
|
| Dam
| 5250
| -
| 5050
|
| 4515
|
| Svet
| 5320
| 5130
| -
|
| 4545
|
| Sar
| 5250
| -
| 5065
|
| 4525
|
| Sar- t500
| 5275
| -
| -
|
| 4550
|
| Sar- t900
| 5275
| -
| -
|
| 4545
|
| Loz
| 5260
| shoulder
| 4525
|
| Loz- t375
| 5260
| 5100
| -
| 4990
| 4525
|
| Loz- t500
| 5270
| -
| -
|
| 4545
|
| Loz- t700
| 5275
| -
| -
|
| 4545
|
| Loz- t900
| 5270
| -
| -
|
| 4545
|
| Bor
| 5260
| -
| 5075
|
| 4525
|
| Bor- t475
| 5270
| shoulder
| 4545
|
| Bor- t700
| 5265
| -
| 5075
|
| 4545
|
| Bor- t900
| 5260
| -
|
|
| 4545
|
Note: the samples denoted with t are heated at corresponding temperature
In the spectra of the investigated obsidians and perlites heated at high temperature where the most part of water is lost, only the maximum at about
3450 cm-1 is observed and in the NIR spectra only at about 4545 cm-1. Therefore the absorption maximum at about 3450 cm-1 can be attributed to the fundamental OH vibrations of hydroxyl groups bounded to the glassy structure. Moor and McMillan (1956), Adams (1961) and Bartholomew et al. (1980) also make such an assignment while Ziborova (1981) attributes this absorbance to OH vibrations of molecular water, weakly bound to the glassy matrix. In the spectrum of scapolite, one of the rare testosilicates with OH in the structure, the same maximum is observed (Fig.1).
Most difficult is the interpretation of the shoulder at about 3220-3240 cm-1. Taking into account the fact that this shoulder is better resolved in the high water containing perlites (Fig.1 - samples Svet, Loz and Bor) and it disappears after heating, it can be assumed that this maximum is produced by OH vibrations of molecular water species, strongly bound to the glass structure (Ziborova, 1981).
Generally, the strong asymmetric absorption at 3200-3600 cm-1 characterizes, from the chemical point of view, weak hydrogen bonds (Novak, 1974). On the basis of published experimental correlation diagrams between the fundamental OH stretching and the length of the hydrogen bonds in water-containing compounds (Nakamoto et al., 1955; Novak, 1974) it is possible to estimate their average bond lengths in the investigated volcanic glasses although much of the compiled data is not for silicates. using these correlations the H2O molecules with absorption at about 3600 and 3240 cm-1 would have average R(O...O) distance 3.1 and 2.8Å (the latter is of ice type H-bond), respectively, i.e. these two types of molecular water are connected with the glass structure in the different way. The absorption maximum at 3450 cm-1 of the hydroxyl groups corresponds to the average R (O...O) distance of 2.9Å. NMR data (Eckert et al., 1987, 1988) also indicate that the OH groups and molecular water species participate in hydrogen bonds with similar distances (2.90-2.93Å for rhyolite glasses). According to the diagrams of Nakamoto et al., (1955) the average O-H distances for the three H- species are in the range 0.94-0.97Å.
Conclusions
The present study confirms that in the obsidians and perlites the different type hydrogen species are present. The following conclusions can be made as the base of our investigations:
1) Analysis of the behaviour of the complex absorption band at 3200-3600 cm-1 in rhyolite glasses with different total water content (obsidians and perlites), as well as comparison of the IR and NIR spectra before and after heating permits a tentative assignment of the observed subbands to be made. The absorption maximum at 3430-3490 cm-1 is attributed
to hydroxyl groups while those at 3590-3595 cm-1 and 3220-3240 cm-1 to two types of molecular water species;
2) Additional weak absorption maximum is observed in the NIR range between 5050-5155 cm-1, which most probably is due to combination models of strongly H-bonded molecular water;
3) The absorption maximum of the hydroxyl groups at about 4545 cm-1 appears to shift slightly to higher frequences after heating, which is, probably, related to the relaxation of their H-bonds;
4) The hydroxyl groups persist in the glass structure even after heating at 1200oC, i.e. pumice formation and industrial expanding of the perlites is due only to the liberation of the molecular water because at these temperatures the diffusion of OH is very low (e.g. at 500oC D OH<0.02D H2O - Zhang et al., 1991). It has to be mentioned once again that the "water" content of perlite in the chemical analyses (H2O+), often determined as loss-on-ignition (L.O.I) at 900-1050oC, gives only the amount of molecular water (Bartholomew et al., 1980);
5) The molecular water is totally released on heating in a large temperature interval. As a consequence of this, the perlites with high water content dehydrate at higher temperatures than those with low water content (Dimitrov et al., 1984). It cannot be excluded, however, that at high temperature the molecular water dissociates by means of the reaction H2O + O = 2OH and remains in the glass as hydroxyl groups;
6) The average R(O...O) distance for the hydroxyl groups is about 2.9Å, while for the molecular water species it is 2.8Å (ice type H-bond) and 3.1Å, respectively. The O-H distance of all type of water species are 0.94-0.97Å.
7) With increasing the "albite" component in the glasses the absorption maximum at 3600 cm-1 shifts to the lower frequencies and R (O...O) distance decreases respectively.
7
Acknowledgements
The measurements for this study have been made in the Laboratory of the Mineralogy and Crystallography (University of Paris YI). We would like to thank: prof. G.Calas for the kind invitation to work in his laboratory with a grant of the French MTR; G. Gouet for measuring the FTIR spectra; Th. Brasset, N. Malengreau, and A. Bedidi for the help in running the NIR spectra; A. Ramos (all from the Laboratory of the Mineralogy and Crystallography of the University of Paris YI) and M. Karadjov (Geological Institute, Sofia) for their help with the thermic treatment of the samples, as well as J.C.Jirard (Laboratory of Magmatic Petrology, University of Aix-Marseille III) for preparations of the doubly polished plates.
References:
- Adams R.V. (1961) Infra-red absorption due to water in glasses. // Phys.Chem. Glasses, vol.2, pp.39-49.
- Bartholomew R.F., Butler B.L., Hoover H.L., Wu C.K. (1980) Infrared spectra of water-containing glass. // J. Amer. Ceram. Soc., vol.63, pp. 483-485.
- Cohen A.J. (1958) The absorption spectra of tectites and other natural glasses. // Geochim. Cosmochim. Acta, vol.14, pp. 279-286.
- Dimitrov V., Yanev Y., Dimitrova-Pankova M. (1984) IR-spectroscopy of Eastern Rhodopes perlites. // Geochim. Mineral. Petrol., Sofia, vol. 19, pp. 86-96 (in Russian with English abstr.).
- Eckert H., Yesinovski J.P., Silver L.A., Stolper E.M. (1988) Water in silicate glasses: Quantitation and structural studies by 1H solid echo and MAS-NMR methods. // J. Phys. Chem., vol.92, pp. 2055-2064.
- Eckert H., Yesinovski J.P., Stolper E.M., Stauton T.R., Hollowey J. (1987) The state of water in rhyolitic glasses. A deuterium NMR study. // J. Non-Cryst. Solids, vol. 93, pp. 93-114.
- Ellis J.V., Lyon W.K. (1936) An interesting infrared absorbtion band in fused quartz. // Nature, vol. 137, pp. 1031-1032.
- Znamenskii V.S. Ed. Genesis Perlitov (Genesis of Perlites (1992) // Moscow: Nauka Publish. House, 190 p. (in Russian).
- Glase F.W. (1955 ) Transmittance of infrared energy by glasses. // Amer. Ceram. Soc. Bull., vol. 34, pp. 291-294.
- Harrison A.J. (1947) Water content and infrared transmission of simole glasses. // J. Amer. Ceram. Soc., vol. 30, 12, pp. 362-366.
- Keller W.D., Pickett E.E. (1954) Hydroxyl and water in perlite from Superior Arizona, Amer. J. Sci., vol. 252, pp. 87-98.
- McMillan P.F., Jakobson S., Hollowey J.R., Silver L.N. ( 1983) A note on the Raman spectra of water-bearing albite glasses. // Geochim. Cosmochim. Acta, vol.47, pp. 1937-1943.
- McMillan P.F., Poe B.T., Stanton T.R., Remmele R.L. (1993) A Raman spectroscopic study of H/D isotopically substituted hydrous alumosilicate glasses. // Phys. Chem. Minerals, vol. 19, pp. 454-459.
- Moore H., McMillan P.W. (1956) Glasses consisting of the oxides of elements of low atomic weight. // J. Soc. Glass. Tech. vol. 40, 193, pp.66-138.
8
- Nakamoto K., Margoshes M., Rundle R.E. (1955) Stretching frequences as a function of distances in hydrogen bonds. // J. Amer. Chem. Soc. vol. 77, pp.6480-6488.
- Nasedkin V.V. (1963) Vodosoderjashie Vulkanicheskie Stekla Kislogo Sostava in Genesis y Izmeneniya(Water-bearing Acid Volcanic Glasses Genesis and Alteration). // Moscow: Acad. Sci. Publish. House, 196 p. (in Russian).
- Nasedkin V.V., Panesh V.I. (1967) Hydroxyl and water in the same varieties of natural and artificial silicate glasses. // Vodnye Vulcanicheskie Stekla y Postvulkanicheskie Mineraly(Water-bearing Volcanic Glasses and Postvolcanic Minerals), Moscow: Nauka Publish. House pp. 26-55 (in Russian).
- Newman S., Stolper E.M., Epstein S. (1986) Measurements of water in rhyolitic glasses: Calibration of infrared spectroscopic technique. // Am. Miner., vol. 71, pp. 1527-1541.
- Novak A. (1974) Hydrogen bonding in solids. Correlation of spectroscopic and crystallographic data // Structure and Bonding, vol. 18, pp. 177-216.
- Orlova G.P. (1962) On the solubility of water in albite melt under the pressure. // Trudy YI Soveshchania po Eksperimentalnoi y Tekhnicheskoi Mineralogii y Petrographii Moscow: Acad. Sci. Publish. House pp. 81-87 (in Russian).
- Scholze H. (1959) Der Einbau des Wassers in Glassern II, Glastechn. Ber., vol. 32, pp. 142-152.
- Scholze H. (1966) // Gasses and water in glass Glass Indust., vol.47, pp. 546-551, 622-628, 670-675.
- Silver L.A., Ihinger P.D., Stolper E. (1990) The influence of bulk composition on the specification of water in silicate glasses. // Contrib. Miner. Petrol., vol. 104, pp. 142-162.
- Stolper E. (1982) Water in silicate glasses: An infrared spectroscopic study. // Contrib. Miner. Petrol., vol.81, pp. 1-17.
- Stolper E. (1989) Temperature dependence of the speciation of water in rhyolitic melts and glasses. //Am. Miner., vol. 74, pp. 1247-1257.
- Yanev Y. (1987) Characterization of volcanic glasses from the Eastern Rhodopes. //Bulgaria, Second Inter. Conferen. on Natural Glasses, Praha: Univ. Karlova, pp. 129-138.
- Zhang Y., Stolper E.M., Wasserburg G.J. (1991) Diffusion of water in rhyolitic glasses. // Geochim. Cosmochim. Acta, vol. 55, pp. 441-456.
- Ziborova T.A. (1981) State of water and hydroxyl in natural glasses after IR spectroscopic data. //Perlites, Moscow: Nauka Publish. House, pp. 177- 187 (in Russian).
- Zotov N., Dimitrov V., Yanev Y. (1989) X-ray radial distribution function analysis of acid volcanic glasses from the eastern Rhodopes. // Bulgaria, Phys. Chem. minerals, vol.16, pp. 774-782.
- Zotov N., Yanev Y., Epel'baum M.B., Konstantinov L. (1992) Effect of water on the structure of rhyolite glasses: X-ray diffraction and Raman studies. //J. Non-Cryst. Solids, vol. 142, pp. 234-246.
- Zotov N., Boysen H., Romano C., Dingwell D., Yanev Y. (1995) Neutron diffraction study of feldspar glasses. Mixed alkali effect. // J. Non-Cryst. Solids (in press).
9
Contents Next
 n) of the perlite samples were also used. They were mounted with haraldite on glass substrates. The measurements were carried out in transmission geometry using selected areas of pure volcanic glass on the FTIR Nicolet 5DX spectrometer. The spectra were recorded only between 4000 and 3420 cm-1 because at lower frequences the spectrum is masked by the absorption bands of the haraldite. The doubly-polished plates were measured in the NIR range also. The spectra obtained do not differ from the corresponding powder spectra.
n) of the perlite samples were also used. They were mounted with haraldite on glass substrates. The measurements were carried out in transmission geometry using selected areas of pure volcanic glass on the FTIR Nicolet 5DX spectrometer. The spectra were recorded only between 4000 and 3420 cm-1 because at lower frequences the spectrum is masked by the absorption bands of the haraldite. The doubly-polished plates were measured in the NIR range also. The spectra obtained do not differ from the corresponding powder spectra.




