INTRODUCTION
Recent advances in our understanding of the physical processes of melting and melt extraction in the mantle have arisen through theoretical work (McKenzie, 1984; Klein & Langmuir, 1987; McKenzie & Bickle, 1988; Niu & Batiza, 1991; Watson & McKenzie, 1991; Spiegelman, 1993) and experimental peridotite melting studies (e.g. Jaques & Green, 1980; Falloon & Green, 1987, , 1988; Kinzler & Grove, 1992; Hirose & Kushiro, 1993; Baker & Stolper, 1994). All these studies have been aided by geochemical studies of samples of residual mantle from mid-ocean ridge and ocean island settings. These peridotites record important information about melting and melt extraction in the oceanic mantle which is not easily obtained from melt compositions (Johnson et al., 1990). In particular, they confirm that melting at mid-ocean ridges and ocean islands is the result of adiabatic decompression of the mantle and demonstrate that melting is fractional or near-fractional.
In contrast, our physical models for melt generation above subduction zones are less well developed. This is in part because physical parameters such as the advection of mantle and melt in the mantle wedge are poorly constrained, and in part because there is a dearth of `wet' peridotite melting experiments from which to study the composition of subduction zone melts and residues. Recently, attempts have been made to model the volume and composition of melts generated above subduction zones (e.g. Plank & Langmuir, 1988; Davies & Bickle, 1991; Davies & Stevenson, 1992; Pearce & Parkinson, 1993; Stolper & Newman, 1994). However, there are considerable differences between these models and hence in the predictions they make for the composition of the residual sub-arc mantle. The models of Davies & Bickle, (1991) and Davies & Stevenson, (1992) predict that the residual mantle should be moderately depleted (8-10% melting) in those incompatible elements not brought into the source region from the underlying subducting plate. In contrast, the decompression melting models of Plank & Langmuir, (1988) and Pearce & Parkinson, (1993) predict a wide range of depletion in the residual mantle from moderate (~10% melting) in arcs initially built on thick crust, to large (20-30% melting) in arcs initially built on thin crust, because the crust is thought to limit the extent of melting beneath arcs. In all models, residual peridotites may be variably enriched in elements derived from aqueous fluids and/or melts from the subducting plate.
Melt percolation and melt-mantle interaction are potentially important processes in modifying the composition of the mantle and its resultant melts (Spiegelman & McKenzie, 1987; McKenzie & O'Nions, 1991; Pearce & Parkinson, 1993). Kelemen et al., (1990, , 1992) suggested that melt-mantle interaction may be a dominant process within the mantle wedge such that the compositions of both magmas and residual peridotites are functions of the amount or style of melt-mantle reaction. Possibly the only consensus view in all these models is that water is likely to be brought into the source region for melt generation, and therefore that peridotites from subduction zone settings are likely to be more oxidized than those from other oceanic settings, by about 2 log oxygen fugacity units (e.g. Wood et al., 1990).
To date, the vast majority of oceanic peridotites sampled and analysed are `abyssal' peridotites from mid-ocean ridge settings (e.g. Bonatti & Michael, 1989; Dick, 1989; Johnson et al., 1990; Niu & Hékinian, 1997b). Only a limited number of oceanic peridotite samples linked to subduction have been studied in detail. We term these supra-subduction zone (SSZ) peridotites (Pearce et al., 1984); a grouping that incorporates peridotites from both island arcs and spreading centres above subduction zones. SSZ peridotites reported in the literature include those from the Puerto Rico Trench (Bowin et al., 1966), Ichinomegata (Takahashi, 1980), the Mariana Trench (Bloomer, 1983; Bloomer & Hawkins, 1983), the Ogasawara Palaeoland (Ishii, 1985), the Tonga Trench (Shcherbakov & Savel'yeva, 1984; Bloomer & Fisher, 1987) and the Luzon arc (Maury et al., 1992). However, few of these studies extend beyond bulk-rock major element analyses and mineral chemistry.
From the preceding discussion, it is apparent that geochemical studies of SSZ mantle peridotites will provide important information on melting and enrichment processes within the mantle wedge. In this paper, we describe the major and trace element geochemistry, and the mineralogy and petrology, of a suite of extant oceanic forearc mantle samples recovered by drilling of two serpentinite seamounts in the Izu-Bonin-Mariana forearc during Ocean Drilling Program (ODP) Leg 125. Specifically, we use data from these peridotites to investigate: (1) the nature of mantle melting in the supra-subduction zone environment; (2) the extent to which SSZ peridotites may be distinguished in the geological record; and (3) the extent to which melt-mantle reactions control the distinctive geochemistry of subduction zone peridotites or magmas.
TECTONIC AND GEOLOGICAL SETTING
The Western Pacific represents the largest intraoceanic destructive plate margin in the world. Figure 1 is a map of a segment of the present-day Western Pacific Ocean south of Japan showing its complex series of basins, troughs, inactive and active ridges, rifts and active island arcs. The region can be broadly divided into two large terranes, one north of 25°N and bounded by the linear Bonin Trench, the other south of 25°N and bounded by the arcuate Mariana trench.

Figure
Bathymetric, seismic, dredging and submersible studies of the Izu-Bonin (Ogasawara)-Mariana forearc between the outer-arc high and the trench axis have led to the identification of a series of serpentinite seamounts where mantle peridotite is exposed on the seafloor (Bloomer, 1983; Bloomer & Hawkins, 1983; Taylor & Smoot, 1984; Ishii, 1985; Fryer & Fryer, 1987). Subsequent drilling during ODP Leg 125 of Conical Seamount in the Mariana forearc (Sites 778-780: Fig. 1, inset a) and Torishima Forearc Seamount in the Izu-Bonin forearc (Sites 783 and 784: Fig. 1, inset b) sampled the suite of peridotites that is the subject of this investigation (Fryer, 1992; Ishii et al., 1992; Parkinson et al., 1992).
ODP drilling in the Western Pacific has confirmed that subduction began in the Eocene at ~45 Ma, initiating volcanic activity, much of it of boninitic character, along the whole length of the Izu-Bonin-Mariana system [see reviews by Pearce et al., (1992) and Taylor, (1992)]. The pre-subduction basin (the West Philippine Basin) was trapped to the west of this `proto-arc'. The arc-basin system then evolved through a series of arc construction, arc rifting and back-arc spreading events to give the present-day geometry depicted in Fig. 1. The rocks associated with the inception of subduction are thus now split, with one part located in the forearc terranes to the east (where the peridotites crop out) and one part located at the eastern margin of the West Philippine Basin to the west.
Information obtained from dredging of the forearc terranes at the eastern margin indicates that they underwent a complex tectonic history. A wide variety of rocks has been recovered, including mantle peridotite, various cumulate rocks and volcanic rocks with boninite, island arc tholeiite, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) affinities (Bloomer, 1983; Bloomer & Hawkins, 1983). The MORB and OIB samples are thought to represent mainly accreted material from the Pacific plate, the MORB from Pacific oceanic crust and the OIB from seamounts, although some may represent West Philippine Basin crust that formed before subduction initiation (Bloomer, 1983; Johnson & Fryer, 1990). The island arc tholeiite and boninite series rocks are thought to represent mainly the crust of the Eocene `protoarc'.
The peridotites discussed in this paper could therefore have three possible origins. They could represent: (1) mantle that existed before subduction initiation and formed part of the basement of the Eocene `protoarc'; (2) the mantle residue from Eocene boninite or island arc tholeiite magmatism within the `protoarc'; or (3) upper mantle from the Pacific plate accreted into the forearc terrane during Eocene-Recent subduction. These alternative origins will be evaluated in this paper.
SAMPLING AND ANALYSIS
Sampling
As noted above, the peridotite samples discussed in this paper come from two serpentinite seamounts drilled during ODP Leg 125. The southerly seamount, Conical Seamount, is located at 19°35'N, 146°40'E, 80 km west of the Mariana trench axis and 30 km above the underlying subducting Pacific plate (Fig. 1, inset a). It is 1500 m high and 20 km in diameter and has a conical shape. Seismic studies of the seamount and surrounding areas indicate that the seamount is located at the intersection of two fault zones (Fryer et al., 1990b). Several small horsts and graben are also located near the seamounts, and this indicates that the area is under active extension. Reflection profiles indicate that the seamount is draped with thin flows, which have been shown from drilling to be composed of serpentinite mud (Fryer et al., 1990b). Two flank sites (Sites 778 and 779) and one summit site (Site 780) were drilled and a variety of clasts including serpentinized harzburgites and dunites were recovered from the serpentinite mud matrix. Cold, dense, high pH (~11) fluids are currently seeping from aragonite chimneys on the summit of the seamount, and geochemical studies indicate that a component of these fluids is derived from dehydration reactions in the downgoing Pacific plate (Mottl, 1992).
The northerly seamount, Torishima Forearc Seamount, is located at 30°55'N, 141°47'E, 30 km east of the Bonin (Ogasawara) trench axis and 20 km above the underlying subducting Pacific plate (Fig. 1, inset b). The seamount is 1400 m high and 20 km in diameter (Ishii, 1985; Kobayashi, 1989). It is mantled by a 1 km thick sedimentary sequence which has no internal reflectors and is thought to be composed of serpentinite muds overlying a substrate with strong internal reflectors (Horine et al., 1990). Gravity models for the Izu-Bonin outer forearc indicate that the seamount must be underlain by low-density material (<2690 kg/m3) down to the décollement and that this material is serpentinized ultramafic rock (Horine et al., 1990). ODP Leg 125 drilled two sites on this seamount, an upper flank site (Site 783) and a lower flank site (Site 784) (see Fig. 1, inset b). In contrast to Conical Seamount, Torishima Forearc Seamount is not actively venting fluids and is not therefore at present undergoing hydration from the underlying subduction zone.
Whole-rock analysis
Representative samples from the various drill holes in the two seamounts were crushed in an agate Tema mill. One aliquot was analysed for major and some trace elements (Sc, Ti, V, Cr, Co, Ni, Cu, Zn and Sr) by X-ray fluorescence (XRF) using a Philips PW 1500 spectrometer at the University of Durham. Extended count times were used for trace elements because of their low concentrations in the peridotites. Replicate PCC-1, DTS-1 and in-house ultramafic standards show that both precision and accuracy are about ±2% for MgO and SiO2; about ±1% for other major elements, Cr and Ni; and about ±5% for Ti, V, Co, Zn and Sr. Cu is commonly below the detection limit of 3 ppm.
A second aliquot was analysed for ~40 elements, including Ti, Ga, Rb, Sr, Cs, Ba, Y, the rare earth elements (REE), Zr, Hf, Nb, Ta, Pb, Th and U by inductively coupled plasma mass spectrometry (ICP-MS) on a VG Elemental Plasmaquad II at the Research School of Earth Sciences (RSES), Australian National University (ANU). The technique has been described by Eggins et al., (1997). Another study by the first author (Parkinson et al., 1998), where peridotites were analysed by ICP-MS at RSES and isotope dilution thermal ionization mass spectrometry (ID-TIMS; for REE) at Royal Holloway University, indicated that the methods of Eggins et al., (1997) are suitable for depleted peridotites and give meaningful results. Although spinels do not always dissolve during the digestion process, they contain very low incompatible element contents and make up a small amount of the mode so that their resistance to dissolution has little effect on the data.
To determine the precision and accuracy of the ICP-MS data, USGS depleted peridotite standards, PCC-1 and DTS-1, were analysed together with procedural blanks during each run. Precision estimates based on between-run reproducibility of PCC-1, DTS-1 and Leg 125 samples are given in Table 2 (see below). The very low level middle rare earth elements (MREE), Y, Rb and Sc have the worst precision (typically ±20-30%) and, for the rest, a precision of ±10% is common. A further check on the MREE-HREE (heavy REE) data is provided by the five samples prepared onboard the JOIDES Resolution and analysed by ID-TIMS (Parkinson et al., 1992). These have a precision of ±2% for all these elements (Thirlwall, 1982), and patterns generated by the two techniques are similar and within the ICP-MS errors estimated above. Light REE (LREE) data on these five samples are not used, however, because samples prepared on the JOIDES Resolution had anomalously high La and Ce values which can probably be attributed to shipboard contamination by the La oxide used in XRF preparation and/or sample contamination during preparation of the powders.
Mineral analyses
Major and minor element analyses of mineral phases were collected on a Camebax Microbeam at RSES by the senior author using energy dispersive techniques (Ware, 1991), with an accelerating voltage of 15 kV and a sample current of 5 nA. These data have been combined with the electron microprobe data of Ishii et al., (1992) and analytical techniques are provided in that paper.
The spinel analyses performed at RSES were specifically analysed for use in oxygen fugacity (fO2) determinations. Wood & Virgo, (1989) argued that a systematic error in the calculated Fe3+/[Sigma]Fe content of spinels is generated with differing atomic Al/(Al + Cr) of the spinel (because of the large error on Al2O3). They advocated the use of samples which have ferric iron determined by Mössbauer spectroscopy as secondary standards. In this study, four of Wood & Virgo's standards (8311, 8315, 8316 and 79-1), which have a range of atomic Al/(Al + Cr) ratios, were analysed at the start of each session of electron microprobe (EMP) analyses, with eight to ten analyses of each grain conducted. Encouragingly, the XFe3O4 calculated on the basis of AB2O4 stoichiometry has similar precision to that reported by Wood & Virgo, (1989) of ~±0·003. However, systematic errors were found in the calculation of XFe3O4, the amount varying from day to day in accordance with the study of Wood & Virgo, (1989), and the ferric iron of the spinels is suitably corrected.
Trace element analyses of the minerals in five representative polished thin sections were conducted on a Cameca IMS-3f ion microprobe at Tsukuba University, Japan (analyst K. T. M. Johnson). These have already been published (Parkinson et al., 1992).
PETROLOGIC CHARACTERISTICS
Table 1 provides a summary of the modal mineralogy, petrographic features and mineral compositions of analysed peridotites. It indicates that they are variably serpentinized and comprise harzburgites with subsidiary dunites. For the harzburgites, the original modal mineralogy is 65-87% olivine, 10-27% orthopyroxene, 0-4% clinopyroxene and 0·2-2·9% spinel. The average dunite has 97-99% olivine and 0·6-2·4% spinel, excluding one sample which contains significant orthopyroxene. Amphibole is present in some samples but rarely exceeds 1% of the mode.

Table 1. Modal abundancies, petrographic information and a summary of mineral chemistry for the Leg 125 peridotites analysed for this paper and for samples analysed by ID-TMS by Parkinson et al. (1992)
Olivine is the most serpentinized mineral in the peridotites and, in some samples, is completely serpentinized to mesh-textured lizardite. However, remnants of fresh olivine are found in most thin sections. Olivine commonly forms elongated and aligned porphyroclasts [terminology from Mercier & Nicolas, (1975)] which form the principal foliation within the peridotites. The porphyroclasts can be up to 10 mm long and 5 mm wide, although smaller grains (~2 mm) are also common. Olivine also forms small neoblasts (0·1-0·5 mm) which are principally found at the grain boundaries of both olivine and orthopyroxene porphyroclasts and have lobate grain boundaries. Olivine compositions range from Fo0·905 in the least depleted (most clinopyroxene-rich) peridotites to Fo0·941 in the most depleted (Tables 1 and Table A1; Ishii et al., 1992).
Orthopyroxene is generally found as porphyroclasts of varying size ranging from 1 to 10 mm along their long axes and up to 5 mm across, and is occasionally altered to bastite serpentine. Unlike olivine and spinel, orthopyroxene is generally not elongate in the direction of the principal foliation although the cleavage planes are often aligned and grains may be in optical continuity. A distinct feature of many of the orthopyroxene crystals is their lobate grain boundaries. In certain samples, this lobate texture is extreme and fresh olivine neoblasts are commonly found within these embayments. The lobate grain boundaries are interpreted as resorption features associated with the incongruent melting or dissolution of orthopyroxene. Exsolution lamellae of clinopyroxene are common within orthopyroxene grains. Orthopyroxene grains show obvious signs of both ductile and brittle deformation. Compositionally, the orthopyroxenes are magnesian with low contents of CaO (<0·2 wt % in clinopyroxene-poor peridotites to an average of 0·6 wt % in clinopyroxene-rich peridotites) and Al2O3 (usually 1-2 wt % and rarely exceeding 3 wt %) (Tables 1 and A2; Ishii et al., 1992).
Clinopyroxene makes up a very small part of the mode of the Leg 125 peridotites. Estimates based on petrographic observations give between 0 and 2% clinopyroxene, whereas those based on least-squares analyses are slightly greater because orthopyroxenes used in the least-squares calculation have exsolved clinopyroxene. Although volumetrically unimportant, the clinopyroxenes have distinct chemical and textural features which play a key part in understanding the petrogenesis of the Leg 125 peridotites.
The peridotites contain several types of clinopyroxene. One has strongly lobate grain boundaries and is not associated with orthopyroxene, and probably represents a residual phase during mantle melting (Menzies, 1973). Some of this primary clinopyroxene is also associated with spinel grains and may record the incongruent melting of clinopyroxene to form spinel. A second type forms small blebs on orthopyroxene grain boundaries and is the product of exsolution. A further type (more common in Torishima Forearc Seamount peridotites where it is associated with amphibole) commonly has thin `tails' and is generally aligned oblique to the principal foliation: this type may be precipitated from percolating melts and/or aqueous fluids. Finally, there are rare, prismatic crystals similar to those described by Kimball et al., (1985) and thought to have formed by recrystallization during serpentinization at ~400°C [see fig. 3 of Evans, (1977)].
Primary clinopyroxenes have a fairly restricted range in mg-number [atomic Mg/(Mg + Fe2+)] of 0·946-0·961 and very low Al2O3 contents of 0·90-2·50 wt % ( Table A3; Ishii et al., 1992). TiO2, Na2O and K2O contents are less than 0·05, 0·10 and 0·01 wt %, respectively. Cr2O3 contents are low, with values never exceeding 1·0 wt % and with rim compositions as low as 0·05 wt % in clinopyroxenes close to spinels. In contrast, Al2O3 and Cr2O3 contents of clinopyroxenes recrystallized during serpentinization are always <0·10 wt %.
Spinel is a ubiquitous phase in the Leg 125 peridotites with a modal abundance of 1-2% in harzburgites and up to 3% in some of the dunite samples. In many of the harzburgites, the spinels are aligned along the principal foliation of the peridotites and are commonly highly deformed and strung out within the olivine matrix. Where the spinels are less deformed, they have anhedral to subhedral rhomb shapes. Relatively undeformed, euhedral, rhomb-shaped spinels are most common in the dunite samples where they may have crystallized from a melt or formed by melt-mantle interaction. Spinels associated with orthopyroxenes have lobate grain boundaries and olivines are occasionally included in spinel grains. Some spinels have ferrit-chromit rims and, in highly serpentinized samples, complete spinel grains have been altered to ferrit-chromit and magnetite. These spinels may become corroded to much smaller (<0·5 mm) rounded grains, and blue Cr-rich chlorite is commonly found distributed in the lizardite around such corroded spinels.
The spinels exhibit a wide variation in cr-number [atomic Cr/(Cr + Al)] from just over 0·3 to nearly 0·9. Spinels in peridotites from Conical Seamount exhibit almost the full range of cr-number whereas those from Torishima Forearc Seamount have the much more restricted range of 0·45-0·60 (see Fig. 2, Tables 1 and A4; Ishii et al., 1992). This difference may, in part, reflect the greater amount of core recovered for Conical Seamount, although there are other, more fundamental, differences that should be independent of sample size (i.e. the mineral chemistry). The Fe2O3 content of spinels in harzburgites from Conical Seamount rarely exceeds 2 wt % and can be as low as 0·1 wt %, whereas spinels in harzburgites from Torishima Forearc Seamount have between 2·5 and 5 wt % Fe2O3. Dunites from Conical Seamount have much higher ferric iron contents than their spatially related harzburgites, with Fe2O3 contents up to 4·25 wt %. These differences are examined more closely in the section on oxygen fugacity.

Figure
Amphibole is found in some of the peridotites but rarely exceeds 1% of the mode. Texturally, two forms of amphibole have been found. Euhedral tremolite grains characterize peridotites from both seamounts that show other evidence of high-temperature alteration such as the presence of antigorite, and these amphiboles are therefore likely to be high-temperature alteration products. Hornblendes have been found in harzburgites from Torishima Forearc Seamount and in one dunite sample from Conical Seamount. In both seamounts, the hornblendes form discrete grains, whereas hornblendes in abyssal peridotites form reaction rims around clinopyroxenes (Kimball et al., 1985). We believe that the former represent magmatic amphiboles, whereas the latter have a hydrothermal origin (Kimball et al., 1985).
Compositionally, the hornblendes are calcic [(Ca + Na)b > 1·34] and plot in the fields of magnesio-hornblende and edenitic hornblende depending on Na content (Leake, 1978). They are magnesium-rich with mg-number between 0·928 and 0·961, and have appreciable chromium and aluminium contents (1·93-2·52 wt % Cr2O3 and 7·50-10·22 wt % Al2O3) and low TiO2 (<0·15 wt %), Na2O (1·30-1·90 wt %) and K2O (<0·20 wt %) contents ( Table A5; Ishii et al., 1992). The tremolite has a very high mg-number (~0·96) and low alumina contents (1-2 wt %).
In one highly serpentinized dunite (779A 11R-1 94-96 cm), two small grains of phlogopite were identified. These are magnesium rich with mg-number between 0·946-0·952, low sodium contents (0·22-0·59 wt % Na2O), 8·82-9·32 wt % K2O and very low TiO2 (<0·08 wt %) ( Table A6). Careful investigation of over 50 thin sections has failed to find phlogopite in any other samples.
Lizardite and chrysotile are ubiquitous secondary minerals in the Leg 125 peridotites. Antigorite, the high-temperature serpentine polymorph, has been observed in several of the Leg 125 peridotites, principally in samples that also contain amphibole or show evidence for extensive hydrothermal alteration. Antigorite forms colourless, fibrous splays within olivine grains and is interpreted here as a retrograde reaction mineral. The secondary mineralogy of the Leg 125 peridotites has been summarized by Fryer, (1992).
TEXTURAL CHARACTERISTICS
Many of the peridotites record a high-temperature foliation which is not significantly different from those described in many ocean floor and ophiolitic peridotites. Girardeau & Lagabrielle, (1992) argued that this early fabric was probably generated in the asthenosphere beneath a spreading centre. They identified two stages of deformation overprinting this high-temperature fabric: first, a high-temperature, low-stress (0·02 GPa) homogeneous episode which generated the porphyroclastic texture observed in many samples; and second, a high-stress (~0·05 GPa) heterogeneous event producing dynamically crystallized olivines in shear zones. This second event started at high temperatures and continued to lower temperatures to the point where evidence for brittle deformation is observed (Girardeau & Lagabrielle, 1992; this study). Importantly, this second event involves the high-temperature quasi-hydrostatic recrystallization of olivine indicative of high water-pressures. This, coupled with the resorption features of the orthopyroxene and the clinopyroxenes that may be associated with interaction of residual mantle with melts and aqueous fluids, emphasizes the importance of fluids in the evolution of the peridotites.
GEOCHEMICAL CHARACTERISTICS
Compositional variations
Bulk rock analyses of 29 representative samples are listed in Table 2. They are presented on a volatile-free basis to reduce the effect of variable element dilution caused by serpentinization. A convenient way to represent the compositional variations is to plot each element, in turn, against MgO. MgO acts here as an `index of depletion' (e.g. Frey et al., 1985), increasing in value as the rock becomes more olivine rich and hence more depleted. It should be noted that MgO is affected by serpentinization but that this effect is small at the scale plotted and the effect on MgO does not seem to be as drastic as those described for abyssal peridotites by Snow & Dick, (1995) with the exception of two samples (778A 2R-1 81-84 and 778A 3R-CC 4-7) which have lost significant MgO and one sample (779A 32R-3 18-22) which has either gained MgO or lost SiO2. The modes of these samples were estimated by point counting as least-squares fitting gave erroneous modes because of the significant modification of the major element chemistry. Of the various elements, some show no significant correlation, because of serpentinization (Cu, Cs, Sr, Rb, Ba, Pb, U), poor analytical precision (Sc, Ba, Th, U, Nb), subsequent interaction between the mantle and aqueous fluid or melt (Zr, Nb, LREE) or because one element is invariant (Mn, Fe, Zn). By contrast, SiO2 gives a good correlation, but this is caused partly by closure.

Table 2. Whole-rock major, minor and trace element compositions
Elements that show good correlation with MgO within the harzburgite from a given seamount are: Si (through closure), Y, Yb (representing HREE), Ca, Al, Ga, V, Co and Ni (Fig. 3). Cobalt and Ni, which are compatible during melting, have positive trends, whereas the other elements, which are all incompatible with respect to olivine, show negative trends with slopes that reflect the degree of incompatibility. These diagrams thus indicate that the total variance contains a significant contribution from the degree of depletion of the rock. This point is further emphasized by the fact that these elements correlate with changes in mineral chemistry indicating that the major element plots cannot simply be explained by variations in the modal abundances of the constituent minerals. We will return to a detailed explanation of these plots in the section on mantle melting.

Figure
Trace elements patterns in peridotites
Chondrite-normalized REE plots for representative samples are presented in Fig. 4. All of the peridotites have very low REE concentrations with HREE concentrations ranging from 0·4 to 0·09 times chondrite, MREE concentrations <0·01 times chondrite and LREE concentrations at around 0·01 times chondrite.
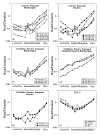
Figure
Although the samples have restricted bulk-rock chemistry, there is a wide variety of REE patterns. The harzburgites have steep, linear, positive HREE-MREE gradients, which generally flatten out at Tb-Gd, and then have flat to upward-inflected LREE patterns. Harzburgites with low orthopyroxene contents can be identified by their more concave-upward HREE-MREE patterns and generally lower absolute HREE contents. Dunites also give patterns that vary from linear to concave-upwards MREE-HREE profiles, and from very LREE-enriched to flat LREE-MREE profiles.
There is some variation between sites. Harzburgites from Hole 780C have steeper HREE-MREE patterns than those from other Conical Seamount sites. Harzburgites from Torishima Forearc Seamount generally have lower HREE contents than harzburgites from Conical Seamount.
The two amphibole-bearing harzburgites from Hole 783A, and the one from Hole 784A, have HREE-MREE patterns which are shallower than any of the other reported peridotites, with a steady decrease in REE content from Lu to La. The effect of the presence of amphibole on the patterns is to enrich MREE-HREE relative to LREE. This indicates that the amphibole present in these harzburgites is not simply an alteration product replacing clinopyroxene but has modified the bulk-rock REE content in accordance with amphibole-melt partition coefficients, and is thus likely to be a primary feature which we interpret as the product of melt-mantle interaction, although its origin as a product of hydrous-fluid metasomatism cannot be ruled out.
Positive Eu anomalies are common features of the rare earth patterns. Given the very low concentrations of the MREE in these samples, however, the origin of these anomalies requires validation. Four observations suggest they are real and primary: (1) the fact that the anomalies were identified by both ID-TIMS and ICP-MS (see Parkinson et al., 1992) suggests that they are not caused by analytical error; (2) the lack of correlation between the size of the anomalies and the content of Ba indicates that it is not an artefact of BaO overlap on Eu in the ICP-MS analyses; (3) the absence of a systematic relationship between LOI and the Eu anomaly suggests that the anomalies are not caused by serpentinization; and (4) our analyses of PCC-1 show the same negative Eu anomaly as those by other workers (Fig. 4). The anomalies show no correlation with calculated oxygen fugacity and there is no plagioclase in any of these samples. Similar positive Eu anomalies have been reported in depleted abyssal peridotites by Niu & Hékinian, (1997b). One possible origin for the positive Eu anomaly is that it reflects a previous melting event, either at a mid-ocean ridge or during the formation of the MORB source when the proto-continental crust was extracted, because Eu2+ is less incompatible than Eu3+ (Y. Niu, personal communication, 1997). The lack of correlation of the anomaly with any obvious geochemical indicators in the Leg 125 peridotite supports the idea that it reflects a previous melting event.
Extended rare-earth patterns are plotted for the same samples in Fig. 5. Here, other incompatible elements are slotted into the REE pattern at a point corresponding to their bulk distribution coefficients during melting. Parkinson et al., (1992) pointed out that the precise order will depend on the fertility of the mantle residue, i.e. the order is different for lherzolites and harzburgites [see also Niu & Hékinian, (1997b)]. In Fig. 5, the order is based on bulk distribution coefficients for melting of a residual (harzburgite) mantle source.

Figure
It is apparent from these patterns that Y shows no anomaly within the MREE-HREE. Ti is commonly enriched with respect to neighbouring REE (Eu and Gd) (the adjacent elements for melting of fertile peridotite) although this is partially masked by the positive Eu anomalies, but slightly depleted relative to Er and Yb (the adjacent elements for melting of strongly residual peridotite). Zirconium and Hf are also commonly enriched both relative to Nd and Sm (the adjacent elements for melting of fertile peridotite) and relative to Eu and Gd (the adjacent elements for melting of strongly residual peridotite). Strontium always has a positive anomaly relative to its adjacent LREE, although the magnitude of this anomaly is very variable. Niobium has both positive and negative anomalies relative to La. The elements U, Th, Ba, Rb and Cs give a spiked pattern on the left-hand side of the diagram in which U, Ba and Cs are enriched relative to Rb and Th.
Trace element patterns in clinopyroxenes
In situ trace element analyses of clinopyroxene, orthopyroxene and olivine from five representative harzburgite samples were published by Parkinson et al., (1992) and key features are summarized in Fig. 6. As expected from the depleted bulk rock composition, the trace element contents of the clinopyroxenes are very low. Chondrite-normalized REE plots for the clinopyroxenes are presented in Fig. 6a and c. Clinopyroxenes from the two Conical Seamount harzburgites (14R-2 and 26R-2) have variably sloping HREE to MREE profiles with an upward inflection of Ce relative to Nd. REE patterns from the two Torishima Forearc Seamount samples have slightly flatter HREE to MREE profiles and only one sample has a Ce inflection. All of the diopsides from the Leg 125 peridotites have lower HREE contents than diopsides from the most depleted abyssal peridotites reported by Johnson et al., (1990), but generally have less fractionated REE, with [Nd/Yb]N ratios of 0·005-0·260 compared with 0·008-0·075 for the most depleted abyssal peridotites.

Figure
The chondrite-normalized multi-element diagrams of these clinopyroxenes (Fig. 6b and d) are marked by large positive Sr anomalies. Strontium concentrations in the clinopyroxenes greatly exceed those in abyssal peridotites with similar modal clinopyroxene contents and/or degrees of serpentinization. This suggests that the positive Sr anomalies in the bulk-rock multi-element patterns (Fig. 5) are in part primary, although from mass-balance arguments a component must also be related to serpentinization, i.e. there is not enough clinopyroxene with a positive Sr anomaly to explain the total Sr content of the whole rocks.
OXYGEN FUGACITY AND GEOTHERMOMETRY CHARACTERISTICS
Oxygen fugacities
To determine oxygen fugacities, we employ the methods of Nell & Wood, (1991) and Ballhaus et al., (1991) based on the reaction
The calculation depends on accurate determination of the ferric iron content of spinel (see analysis section) and gives oxygen fugacities with standard errors of about 0·2-0·3 log units. Temperatures of equilibration can be calculated at an arbitrary pressure of 1 GPa using (1) the Fe-Mg exchange thermometer of
Ballhaus et al., (1991), and (2) the temperature of primary Bonin-Mariana bon- inite magma, which has been estimated as 1250°C (van der Laan et al., 1992). The closure temperature of the reaction should lie between these two extremes, but probably closer to the lower value. Results of calculations based on this lower temperature are given in Table 3, quoted as log units relative to the FMQ (fayalite-magnetite-quartz) buffer [[Delta]log fO2(FMQ)]. Table 3. Calculated oxygen fugacities using the Nell-Wood calibration (Nell & Wood, 1991)
This table highlights fundamental differences in the calculated oxygen fugacities between peridotites from the two seamounts. Harzburgites from Conical Seamount are more reduced, recording oxygen fugacities of [Delta]log fO2(FMQ) = -1·10 to + 0·35 compared with + 0·78 to + 1·59 for harzburgites from Torishima Forearc Seamount. A striking feature of the data is that oxygen fugacities are always highest in dunites from each peridotite suite. Dunites from Conical Seamount record oxygen fugacities of [Delta]log fO2(FMQ) = -0·16 to + 1·24 and those from Torishima Forearc Seamount of [Delta]log fO2(FMQ) = + 1·20 to + 1·81. This observation is important because oxidized dunites from Conical Seamount are in close proximity to reduced harzburgites, indicating that the dunites and harzburgites record different petrogenetic histories. The histogram in Fig. 7a compares the two forearc seamounts with each other and with the abyssal peridotite data set of
Wood & Virgo, (1989) and
Bryndzia & Wood, (1990). Because the abyssal peridotite data were calculated from Dick's `rehomogenized' pyroxene data, which yield high temperatures of 1200-1400°C, the abyssal peridotite data set had to be recalculated using the temperatures obtained from the
Ballhaus et al., (1991) geothermometer to ensure compatibility between the two data sets. The consequence is that the average oxidation state of the abyssal peridotites in this study was modified (for comparison purposes only) to about -0·28 [Delta]log fO2(FMQ), ~0·6 log units more oxidized than the results of the
Bryndzia & Wood, (1990) study. Figure
The histogram demonstrates that the oxygen fugacities of Conical Seamount peridotites lie within the abyssal peridotite (and MORB) range whereas those of Torishima Forearc Seamount peridotites are distinctly higher than abyssal peridotites, and within the supra-subduction zone range. We examine the significance of this finding in the next section.
6Fe2SiO4 + O2 = 3Fe2Si2O6 + 2Fe3O4·


Geothermometry
Of the various geothermometers that can be applied to peridotites the olivine-spinel Fe-Mg exchange thermometers of Ballhaus et al., (1991) and Sack & Ghiorso, (1991) gave the most consistent results and have been used in this paper. For reasons of space the results of other geothermometers are not discussed, although they can be obtained from the authors on request.
Figure 7b is a frequency histogram of temperatures calculated for coexisting olivines and spinels using the Ballhaus thermometer (see Table 3) at the arbitrary pressure of 1 GPa. Temperatures calculated for a number of abyssal peridotites are also shown in this figure. The average temperature calculated is 647 ± 40°C (±1[sgr]) for the Leg 125 peridotites and 806 ± 75°C for the abyssal peridotites. Within a thin section, there may be a range of temperatures represented, with the lowest recorded in small olivine grains close to spinel grain boundaries. Although this accounts for some of the spread in the data, a t-test indicates that there is a highly significant (>98%) difference between the temperatures calculated for the Leg 125 and abyssal peridotites. The displacement of Leg 125 spinel compositions to lower mg-number (Fig. 2) can similarly be explained by a lower closure temperature for the Leg 125 peridotites.
By contrast, the more complicated Sack & Ghiorso, (1991) model yields mean temperatures of 658 ± 49°C for the Leg 125 peridotites and 747 ± 97°C for the abyssal peridotites. Although the abyssal peridotites' temperatures are still nearly 100°C higher, the t-test now indicates that there is no significant difference in the populations at the 95% confidence level. The difference between these two methods may reflect a compositional dependence in the Sack & Ghiorso thermometer, possibly linked to the fact that spinels from the Leg 125 peridotites have, on average, higher cr-number than spinels from the abyssal peridotites.
GENESIS OF THE FOREARC PERIDOTITES
Evidence for the pre-subduction mantle history
Figure 8 is a plot of [Delta]log fO2(FMQ) against cr-number for the Leg 125 peridotites and published abyssal peridotite data. As before, the oxygen fugacity data reported refer, for consistency, to those calculated for subsolidus equilibration temperatures. It is apparent from this plot that, although the cr-numbers of spinels in the two forearc seamounts overlap, the Torishima Forearc Seamount harzburgites have markedly higher oxygen fugacities for a given cr-number. Moreover, the Conical Seamount harzburgites form a trend from the abyssal peridotite field to higher, more subduction-like, oxygen fugacities with increasing cr-number. In contrast, the dunites from both seamounts have subduction-like oxygen fugacities, although the dunites from Conical Seamount have spinels with greater cr-number. The fact that the samples with highest oxygen fugacities are those that contain primary amphibole supports models that require a relationship between aH2O and fO2 (Bryndzia & Wood, 1990).

Figure
The significant feature of this diagram is the single linear trend formed by both the harzburgites and the interlayered dunites from Conical Seamount. At the lower end of this trend are harzburgites with fO2 and cr-number within the abyssal peridotite range; at the upper end are dunites with fO2 and cr-number in the supra-subduction zone range. The obvious explanation is that the dunite end-members formed from supra-subduction zone magma whereas the harzburgite end-member is the residue from melting at a pre-existing mid-ocean ridge. The trend then represents the interaction between the supra-subduction zone magma forming the dunite and the pre-existing oceanic mantle lithosphere. This interpretation can be extended by considering the very high cr-number of the dunite end-member of the mixing-interaction trend. These cr-numbers correspond to those of boninite (as opposed to tholeiite) spinels from the Bonin-Mariana forearc, which are inferred to originate by remelting of oceanic lithosphere (van der Laan et al., 1992). It is thus possible that the harzburgites from Conical Seamount represent the upper-mantle section of a pre-existing oceanic lithosphere from which the boninites were derived.
In contrast, both harzburgites and dunites from Torishima Forearc Seamount are oxidized, with the dunites having slightly higher cr-number. This observation is consistent with the formation of both rock types in a supra-subduction zone setting. The harzburgites may then represent the residues from a melting event and the dunites the products of crystallization from, or interaction of the harzburgite with a magma from the same event. The lower cr-number of the dunites from Torishima Forearc Seamount compared with the dunites from Conical Seamount points to an origin from a tholeiitic, rather than boninitic, melt.
The Leg 125 oxygen fugacity data thus indicate that the mineral chemistry of the two sets of peridotites is controlled by two different processes: at Conical Seamount, supra-subduction zone boninite magma has interacted with oceanic mantle lithosphere unrelated to subduction (i.e. with abyssal peridotite); at Torishima Forearc Seamount, supra-subduction zone tholeiite magma has interacted with mantle lithosphere formed by the same (or another) supra-subduction zone melting event.
Evidence for subduction zone enrichment
The evidence for a supra-subduction zone, as opposed to mid-ocean ridge, ocean island or ocean margin setting, for the forearc peridotites has been demonstrated in terms of oxygen fugacities and cr-number of the spinels using Fig. 8. Although ocean margin peridotites can also have high oxygen fugacities, they rarely also have high cr-number. Elemental confirmation for a supra-subduction zone origin is perhaps best seen in the positive Sr anomaly on the clinopyroxene multi-element diagrams and by a Zr enrichment (Fig. 6). By using clinopyroxene rather than whole-rock data we can be sure that the Sr enrichment is primary, i.e. not linked to subsolidus reaction with aqueous fluids.
Suitable projections with which to illustrate these discriminants are by plotting the Sr vs Nd (Fig. 9a) and Ti/Zr vs Ti (Fig. 9b) in the clinopyroxenes. Neodymium is used because Nd has a similar bulk distribution coefficient to Sr while being significantly less enriched in a subduction component. The plot demonstrates that the Leg 125 peridotites are displaced from the abyssal peridotite trend towards high Sr contents. Titanium is used because it is not enriched in the subduction component while also being a good indicator of degree of melting. Figure 9b illustrates that the Leg 125 peridotites are enriched in Zr compared with abyssal peridotites at a given Ti content. Thus the rocks studied can be interpreted as having a subduction signature. What is not resolved by this plot is how this subduction signature is introduced into the mantle-directly or through melt-mantle interaction.

Figure
Evidence for the degree and nature of partial melting
The modal mineralogy of peridotites can be useful in formulating partial melting models. Most workers (e.g. Jaques & Green, 1980; Baker & Stolper, 1994) agree that, during partial melting in the spinel facies, clinopyroxene will be the phase consumed most rapidly. Therefore, the peridotites will have a range of modal clinopyroxene from ~15% in a fertile (unmelted) peridotite to 0% after ~25% melting. Clinopyroxene may persist to slightly higher degrees of melting if partial melting was initiated in the garnet field as it is a reaction product when garnet breaks down to spinel. Recently, experimental results (Gaetani & Grove, 1998) indicate that clinopyroxene may also persist to higher degrees of partial melting during hydrous melting of a spinel peridotite than is the case for anhydrous melting. Dick & Fisher, (1984) argued that the clinopyroxene content of peridotites is only a measure of the degree of depletion, whereas the forsterite content of the olivine is a measure of the total degree of melting as olivine-melt equilibria are not changed substantially by water (Gaetani & Grove, 1998). On these criteria, all the Leg 125 peridotites are highly depleted (having <2% primary clinopyroxene) and have undergone high degrees of melting (the olivines have high mg-number).
Importantly, many of the peridotites from both seamounts have orthopyroxenes with resorption features and fresh olivine within lobate grain boundaries suggesting that incongruent melting of orthopyroxene has taken place. Phase equilibria studies indicate that melting of this type is restricted to extremely low pressures in anhydrous, iron-bearing systems (<0·14 GPa; Morse, 1980) but that it extends to higher pressure in hydrous peridotite systems. Modal abundances of orthopyroxene in the Leg 125 peridotites vary from 10 to 24% and, although some of this variation is related to sampling errors and tectonism, many of the peridotites have lower orthopyroxene contents for a given olivine content than would be expected for anhydrous melting residues. Therefore, at least some of the melting history may have involved hydrous conditions. It should be noted that Niu, (1997) argued that incongruent melting of orthopyroxene occurs throughout the melting region beneath mid-ocean ridges where melting is essentially anhydrous.
Calculations can help to quantify the partial melting histories of the Leg 125 peridotites. The first point to make is that the variation in the element-MgO plots is not just an artefact of varying modal contents of minerals with the same composition. Figure 10a illustrates cr-number of spinel (a proxy for degree of melting) vs Al2O3 and Yb contents of the whole rock. The good negative correlations, especially for the harzburgite data, indicate that variation in major element chemistry reflects varying amounts of a process (either melting or reaction) not differences in modal mineralogy. We can now model the partial melting histories more quantitatively. As with arc volcanic rocks, the most useful elements are those unaffected by subduction zone metasomatism, i.e. the subduction-conservative elements (Pearce & Parkinson, 1993), most of which give straight lines on the element-MgO plots (Fig. 3). These include: the compatible elements Ni and Co; the slightly to moderately incompatible elements Sc, V, Ga, Al, and the incompatible elements Y, Ti and the HREE. The very highly incompatible elements Zr, Hf, Nb and Ta appear to be too sensitive to melt infiltration and melt-residue interaction to be useful. Of the elements listed, Sc, Y and the HREE are useful because they can help to evaluate the role of garnet in the melting process. Vanadium can additionally provide information on oxygen fugacity.

Figure
For this study, we model these elements using the fractional melting equation for a porosity of 1% and our own compilation of partition coefficients and rates of disappearance of mantle phases (Pearce & Parkinson, 1993), which lie within the 2[sgr] range of the more recent compilation of Green, (1994). First, we can use clinopyroxene data to model the melting. Figure 10b is a plot of Yb vs Ti in the Leg 125 peridotites. This plot illustrates that the Leg 125 data lie at the depleted end of abyssal peridotite melting array at ~25% fractional melting. Another approach is to model whole-rock data. Here we use theoretical patterns of the subduction-conservative elements normalized to fertile MORB mantle (FMM) [this is essentially an N-MORB source which was used to model subduction zone melting processes by Pearce & Parkinson, (1993)], which give patterns that show progressive depletion with element compatibility. Compared with these model patterns, harzburgites from the two seamounts match well the patterns for 15-25% melting (Fig. 10c and d).
Bivariate plots of subduction-conservative element pairs examine aspects of the melting process in more detail (Fig. 10e and f). The plot of Ti vs Yb shows the mantle depletion trends for equilibrium batch melting and fractional melting, and for melting columns with garnet residue and no garnet residue. These data demonstrate that: (1) the data fit the fractional melting trend rather than the batch melting trend, thus showing that the conclusion of Johnson et al., (1990), that abyssal peridotites are the residues from fractional rather than batch melting, also applies to these supra-subduction zone peridotites; (2) a fractional melting model with the partition coefficients used indicates 15-25% partial melting; and (3) there is no clear evidence for any significant garnet residue in the melting process. These data also indicate that for most of the subduction-conservative elements melting in the mantle wedge can be modelled in a similar manner to beneath mid-ocean ridges, with no obvious need for amphibole as a residual phase, except that the Leg 125 peridotites have on average undergone higher degrees of partial melting than abyssal peridotites.
The model is sensitive to the precise values of the partition coefficients mainly in the estimates of the degree of melting. Changing the coefficients could affect the absolute melting estimates by up to 5% (see Fig. 10b), but the estimated differences in degree of melting between Leg 125 and abyssal peridotites would be little changed. It should be noted also that varying the source composition can also affect the absolute melting estimates but that our FMM source is very similar to other estimates of N-MORB sources, but again does not change the relative differences in melting Leg 125 and abyssal peridotites.
The plot of V vs Yb (Fig. 10f) is particularly useful as depletion trends are strongly dependent on oxygen fugacity (Pearce & Parkinson, 1993). For the relatively reducing conditions characteristic of abyssal peridotite formation (e.g. FMQ - 1), the ratio of V3+/(V4+ + V5+) is high and partition coefficients are low. The depletion of V in the mantle residue for a given degree of melting is therefore low. For the relatively oxidizing conditions characteristic of supra-subduction zone peridotite formation (e.g. FMQ + 1), the ratio of V3+/(V4+ + V5+) is low and partition coefficients are high. The depletion of V in the mantle residue is therefore relatively rapid and the depletion trend steeper. Also shown in Fig. 10f is a field for a suite of well-characterized oceanic peridotites which record a simple melting history (Parkinson et al., 1998). Although they plot within the correct section of the diagram for their calculated oxygen fugacity and at roughly the correct degree of partial melting, they define a trend which is much steeper than the FMQ - 1 and FMQ melting trends. This indicates that although the V vs Yb plot is potentially a very useful discriminant of subduction zone peridotites, further experimental work is needed to constrain the melting curves (e.g. Canil, 1997).
In Fig. 10f, Torishima Forearc Seamount harzburgites plot on a trend for oxidized peridotites in the range 20-25% melting. By contrast, the Conical Seamount harzburgites plot on a trend from reduced mantle at 15-20% melting toward the dunites on the oxidized mantle trend. They thus indicate that the Conical Seamount peridotites were the residue from about the maximum amount of mid-ocean ridge melting. The trend to higher degrees of melting may, however, be the result of melt-mantle (boninite) interaction and thus not a true indication of the degree of melting.
Evidence for melt-mantle interaction
The clearest evidence for melt-mantle interaction comes from the linear compositional relationship between the dunites and harzburgites from Conical Seamount (Fig. 8b). The origin of the end-member dunites, as inferred from the oxygen fugacity and chromite data, is that they formed from primary oxidized melts of supra-subduction zone origin. The two mechanisms of dunite formation usually considered are crystal segregation or preferential dissolution of pyroxenes in zones of melt focusing (e.g. Kelemen et al., 1995). However, the latter model is more consistent with the continuous trend between dunites and harzburgites and with the fact that the dunites contain small amounts of tectonized (and hence probably relict) orthopyroxene. In this model, the spinels are oxidized because the original, reduced, spinels have interacted with oxidized subduction zone melts. More recently, Niu, (1997) and Niu & Hékinian, (1997b) have argued that high modal olivine contents of abyssal peridotites occur because of passive crystallization of olivine at shallow pressures. Peridotites described by Niu & Hékinian, (1997b) have increasing modal olivine correlating with decreasing forsterite contents; this feature suggests that the `excess' modal olivine crystallizes from an evolving melt. In contrast, the modal olivine contents of the Conical Seamount peridotites have good positive correlations with cr-number, a weak positive correlation with the forsterite content and a good negative correlation with Al2O3 content of orthopyroxene. All these features are inconsistent with simple passive crystallization of olivine.
Although the HREE and some incompatible trace element contents of the peridotites are consistent with a simple fractional melting model, the most strongly incompatible elements such as the LREE have far higher concentrations than can be explained by such a model. There are several possible explanations. First, melting with relatively large porosities (5%) will cause the most strongly incompatible elements to be retained during melting. However, this will also increase the HREE and Ti contents of both the whole rock and clinopyroxenes to levels above those observed. Another possible explanation is an open system melting model whereby the peridotite re-equilibrates with an incompatible element enriched melt which represents either a melt generated within the melting column (e.g. Johnson & Dick, 1992) or an exotic melt fluxed into the melting peridotite (e.g. Ozawa & Shimizu, 1995). The very depleted nature of these harzburgites means that only a small amount of reacting melt (<1%) would be needed to modify the incompatible element concentration of these rocks if a simple open system melting model is considered.
Alternatively, enrichment in elements such as the LREE is a common feature of many arc lavas (e.g. Hawkesworth et al., 1993) and it is possible that the LREE enrichment in these peridotites is related to melt-aqueous fluid interaction within the mantle wedge [see also Parkinson et al., (1992)]. This is supported by the fact that both whole rocks and clinopyroxenes exhibit LREE enrichment, which indicates that this enrichment took place at high temperatures and is not the product of serpentinization. Moreover, the Zr enrichments in these clinopyroxenes favour enrichment by a silicate melt rather than by subsolidus diffusion. As Zr, Sr and LREE enrichments are a common feature in many of the boninites drilled from the Izu-Bonin-Mariana forearc (Pearce et al., 1992), it is probable that some of the peridotites were involved in boninite genesis or modified by boninitic melts [see also Parkinson et al., (1992) and Pearce et al., (1992)]. However, it is interesting to note that the subduction component calculated for the Leg 125 peridotites by Parkinson et al., (1992) is similar in character to the subduction component calculated by Stolper & Newman, (1994) for a suite of Mariana back-arc basin basalts.
Evidence for subsolidus processes
In theory, closure temperatures (i.e. the apparent temperatures at which observable diffusion stops) provide important information about the cooling, and therefore tectonic histories, of these rocks. Henry & Medaris, (1980) and Dick & Fisher, (1984) both recognized that, on average, abyssal peridotites have higher equilibration temperatures than Alpine peridotites and argued that abyssal peridotites undergo faster cooling because of seawater influx into the peridotites. The results presented in the geothermometry section indicate that closure temperatures for Leg 125 peridotites are lower than those for abyssal peridotites, although the magnitude and significance of the differences depend on the thermometer used. In general, however, very low temperatures are recorded by many Leg 125 peridotites (<650°C) but only the most deformed abyssal peridotites (Jaroslow et al., 1993).
The simple formulation for the closure temperature (Tc) for spherical geometries (Dodson, 1973) allows the cause of such low-temperature equilibration to be examined:
 |
where [Delta]Ha is the activation energy, D0 is a pre-exponential term, A is a geometric factor (55 for a sphere), a is the radius of the grain, R is the universal gas constant and dT/dt is the cooling rate. The choice of values for [Delta]Ha and D0 is derived for the rate-determining diffusion rate, in this case Mg diffusion in a Mg-rich olivine. Valuesused are [Delta]Ha = 444 kJ/mol and D0 = 1·54 * 103 cm2/s (Morioka, 1981). A plot of Tc vs log a, contoured for different cooling rates (Fig. 11a) shows that, for the very low equilibration temperatures calculated for the Leg 125 peridotites, both very small grain sizes (a ~0·01 cm) and very slow cooling rates (dT/dt = 0·1 K/my) are needed.

Figure
Numerical models have also been used for peridotites cooling in an oceanic plate by conductive heat loss. The initial depth-temperature profile is a convective geotherm (e.g. McKenzie & Bickle, 1988). Depth-temperature-time profiles are then calculated using a simple one-dimensional explicit finite difference model (see Peacock, 1989) for a variety of thermal diffusivities. The model calculates the closure temperatures recorded by a depleted peridotite at the top of the melting column (0·3 GPa) with an initial olivine composition of Fo91 and spinel with a cr-number of 0·50. Concentration profiles in coexisting spinel and olivines are calculated using explicit finite difference methods. The diffusion rates used are those of Morioka, (1981) for olivine and Freer & O'Reilly, (1980) for spinel. These values have been extrapolated to much lower temperatures than those at which the experiments were determined and the validity of this step is questionable (see Morioka & Nagasawa, 1990). However, until more diffusion data are available for lower temperatures, extrapolations of this kind are unavoidable.
For both cooling models (constant and exponential), using a realistic thermal diffusivity of 10-6 m2/s, it is difficult to reproduce the very low re-equilibration temperatures calculated unless grain sizes of 0·01 cm persist for substantial periods of their cooling history. Empirical observations and experimental work (e.g. Drury & Van Roermund, 1989) suggest that this is unlikely to happen. Although many peridotites are sheared and contain small neoblasts, peridotites attempt to anneal during cooling and grain growth proceeds until an equilibrium grain size is produced. This process is greatly assisted by fluids in the peridotite (Drury & Van Roermund, 1989). The most likely explanation is therefore that the diffusion rates are substantially in error. An order of magnitude increase in the diffusion rate has an effect of moving the calculated lines down by ~80°C (Fig. 11b). For reasonable cooling rates and grain sizes, the diffusion coefficients must increase by three orders of magnitude to model the data.
Two mechanisms might increase diffusion coefficients by the required amount. First, deformation during cooling may enhance diffusion rate. Sneeringer et al., (1984) found that Sr diffusion is two orders of magnitude faster in natural diopsides than in synthetic ones, indicating that defect densities affect diffusion rates. Even higher defect densities would be expected during deformation, which would further enhance diffusion rates (Kramer & Siefert, 1990). The Leg 125 peridotites are variably tectonized and record evidence of shearing, but there is no indication that they are more deformed than any of the abyssal peridotites recorded in the literature. Second, fluids such as water may also enhance diffusion rates (Freer et al., 1982; Graham & Elphick, 1990). The Leg 125 peridotites have textures which are indicative of high-temperature fluid (water) interaction and many of the peridotites have high oxygen fugacities compatible with high water contents. The most obvious reason for the low equilibration temperatures recorded in these and many other ophiolitic peridotites is the presence of water assisting the diffusional re-equilibration. This feature may be characteristic of supra-subduction zone peridotites, though not diagnostic as it is also exhibited by alpine peridotites (Henry & Medaris, 1980). We think that the Conical Seamount harzburgites were initially formed within the Pacific plate or West Philippine Basin and then were accreted into the forearc and involved in boninite genesis. From their low equilibration temperatures and spinel chemistry they must have cooled through the olivine-spinel blocking temperature within an SSZ environment. We argue that this indicates they were accreted into the forearc while still hot, were then involved in boninite genesis and finally cooled within the forearc.
CONCLUSIONS
ACKNOWLEDGEMENTS
This research was carried out as part of the senior author's Ph.D. thesis research under the tenureship of an NERC (UK) studentship (GT4/89/GS/27). Research costs at Durham were covered by an NERC Ocean Drilling Special Topics research grant (GR3/416) to J.A.P. We thank Drs Elaine McPherson, Martin Menzies and David Hirst for constructive comments on earlier aspects of this work. Yaoling Niu and an anonymous reviewer had the onerous task of reviewing the paper twice and provided numerous useful suggestions which improved the manuscript. Dr Paul Sylvester is thanked for his patience and persistence in undertaking the ICP-MS analyses at RSES, ANU, and Nick Ware for assistance with microprobe analyses at the ANU. John Miller aided and abetted resampling the Leg 125 cores at the Gulf Coast Repository at Texas A&M University. Tony Ewart is thanked for his remarkable patience during the various revisions of the manuscript. Research costs at the ANU were defrayed by a large ARC grant to Professor Richard Arculus. This is GEMOC Publication 97.






