I. Magmatic systems and petrology
of the silicate melts
Zharikov V.A. Problems of granite genesis.
The experimental data on the different aspects of the granite genesis were summarised. There are many vital conclusions, among which the following are most important.
1. Granitization can be caused by mantle fluids, in which the solubility of silica and alkalies significantly decreases under decompression and on interaction with sialic material.
2. Fluid is an additional phase to eutectics regarding other minerals (Qtz, Pl, Kfs); therefore, some minerals and monomineral aggregates are commonly replaced with eutectic melt.
3. Eutectic melts slightly depend on the composition of silica-rich rocks.
4. Eutectic melt composition is significantly affected by pressure, PH2O, and activities of KOH, NaOH, HCl, and other perfectly mobile components (Zharikov, Epelbaum, Bogolepov, 1994).
The computer modelling of the interaction between mantle basalt melts and sialic crust have revealed many important features of this process. The most significant are: occurrence of secondary acid magmatic sources at the roof and floor of the basalt chamber; possibility of basic-to-acid, acid-to-basic, and contrast magmatism; liquid hybridization; monotonous differentiation and normal magmatism (up to andesite-dacite) at the closed-system conditions with contamination at the early stage (Zharikov et.al, 1991, 1994).
References:
Epelbaum M.B. and Simakin A.G. Theoretical and experimental study on the processes of melting and formation of granite magma.
The theoretical study has been carried out on the processes of melting and formation of granite magma at the melting of water-bearing acid rocks resulted from their interaction with the basalt chamber. The borderline conditions of the equality between the fluid pressure and pressure in the magma have been considered. The calculations showed that an essential filtration fluid transfer was possible even at rather low permeabilities. Under the conditions of earth crust melting a relatively open system forms automatically and a transfer to close systems takes place at K=10-22 m2. Nevertheless it can be supposed that a degree of rocks alteration at the melting front due to the massive fluid flows is not high, because these flows accumulate in the melt and are comparable with the flows liberating at crystallization (Simakin, Epelbaum, 1995).
The program of modeling melting processes as well as crystallization and convection of the main melt by the method of intermediate field was proposed to calculate the melting rate in the granite medium with account of convection (jointly with O.Zatsepina, IEPh RAS). The simplified presentation of the two-dimension flow is used in this method. The solution is to be found relative to vertical distributions of temperature, concentration, convection velocity. The rate of the convection heat exchange is high, therefore, the crystallization front (as a border of complete consolidation) at many parameters appears only at the starting time period. Further, the melting of already formed crystalline layer and closing of the main melt with the granite one ( or the beginning of the latter formation ) takes place. Taking account of convection provide a higher yield of the granite melt due to a decrease in heat dissipation. The melting of the granite substrate is considered in detail at the contact with the main melt at the conductive heat exchange. In the one dimension model the borders of melting regimes were found without crystallization of the overheated main melt, melting followed by the crystallization of the main melt and crystallization of the main melt without melting of an acid substrate. Two dimension calculations of melting and assimilation of an acid melt by the basic one with changing melting diagram were carried out. Convection and mixing are supposed at the powdering of the acid melt in the main chamber. The volumes and composition of the hybrid melts were estimated which showed the reality of the several ways of granite melts formation. One of these ways is formation of granite melts at their accumulation and movement in the roof of the main intrusive. The other way is at the fractional crystallization of the hybrid melts (Zharikov et al., 1994).
References:
10
Simakin A.G. Dynamics of the magmatic chamber crystallization.
A dynamic model of the magmatic chamber has been developed which allows for mechanical properties of the wall rocks, state and water saturation of the magmatic melt, peculiarities of the crystallization processes evolution. The model illustrates and allows to analyze the factors and conditions of the formation of different fracture sets and extenuated dynamic zones through which the flow of magmatogenic fluid and ore-bearing solutions goes (Simakin, Khudyaev, 1992).
An experimental and theoretical study has been carried out on the process of alkaline feldspar growth. We managed to associate the known ideas on the dependence of crystal growth rate on the saturation with our theoretical data: the model of mass exchange at the top of the prolonged crystal, the diffusion on viscosity dependence at essential undercooling, and to describe experimental data on growth rate of Fsp from the water bearing (from 2 and 4 wt %) granite melt in full volume. A deviation from the linear dependence of growth rate on reverse viscosity was noted at high undercooling. This effect correlates well with the contravention of the Stocks Einstein law we revealed earlier. The similar effects are found in the experimental data of Muncill and Lasaga, 1987, 1988. The dependence of the forms of feldspar growth from the melt on the undercooling degree and melt viscosity has been analyzed (Simakin, Chevychelov, 1995).
Within the study on the dynamics of intrusive crystallization the problems of the structure of upper boundary layer and sedimentary convection were accentuated and solved. An earlier proposed method of the complex solution of the problem of crystallization in the two component system with an eutectics and crystals precipitation in equilibrium approximation has been used for the approximated solution of the same problem in kinetic approximation. The distribution of precipitating crystals by sizes in accordance with the Stock's relation is taken into account in the solution. It is valid for a fast growth kinetics. In this case the deviations from the equilibrium can be considered as negligible and equilibrium approximation of the volume crystallization rate can be used. Kinetic equation for crystal size distribution is solved in common with equilibrium limitations for the sum volume rate of the crystallization. The method is simple and allows to estimate the parameters values at which crystals cease being captured completely by the solidification front and sedimentary fractionation becomes possible (Simakin, Trubitsyn, 1995; Simakin, Trubitsyn, and Schmeling, 1994).
The stationary form of sedimentary convection which is an essential factor of heat- and mass- exchange at the melt solidification in the magmatic chamber was theoretically studied. The rate dependence of the descending liquid flows enriched in crystals from the sedimentary Relei number was calculated, the form of the closed liquid flow was obtained. The predicted picture was observed experimentally at the laboratory investigation of this phenomenon in Bayrenth, Germany (Simakin, Schmeling, Trubitsyn, 1995).
References:
Zharikov V.A. and Khodorevskaya L.I. The experimental study of amphibolite melting.
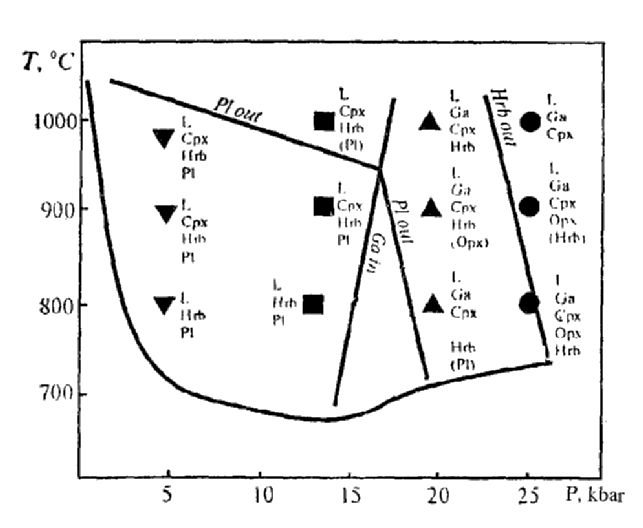
Experiments on amphibolite melting were carried out at pressures within 5-25 kbar, at dry and water-saturated (at water pressures 5 and 7.5 kbar) conditions in order to clarify the possibility of acid magma melting in the subduction zone. The following main regularities were established by the study of the compositions of melt and restite coexisting phases (phase compositions of the experimental systems are shown in Fig.1).
The silica-saturated melts are produced from the olivine-normative amphibolite by incongruent melting of hornblende within the whole pressure range studied (5-25 kbar). The partial melt compositions depend on temperature (melting degree) and pressure as shown at the diagram of normative An-Ab-Ort compositions (Fig.2). Sodium-series melts, corresponding to tonalite-trondhjemite composition, form at moderate pressures (5-14 kbar), whereas the more potassic (granite-granodiorite) melts occur at higher pressures (20-25 kbar). The amphibolite melting in water-saturated systems differ slightly from that in "dry" systems (when water is fixed only in amphibole). This experiment indicates the failure of the "dehydration-melting" mechanism. Experiments performed confirm the possible formation of granitic melts from olivine-normative rocks. At least the amphibolite-facies metamorphism necessary for this process takes place.
11

Fig.1. Summarised experimental T-P diagram of amphibolite melting: M - melt, Pl - plagioclase, Hbl - hornblende, Cpx - clinopyroxene, Opx - orthopyroxene, Ga - garnet. Solidus lines at P=PH2O; lines indicating appearance (in) and disappearance (out) of minerals in restites.
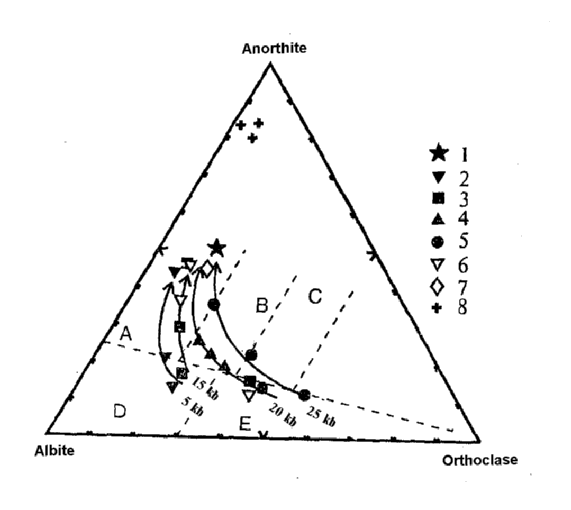
Fig.2. Diagram of normative Ab-An-Or compositions of the partial melts: 1-initial amphibolite composition; Resulting compositions: 2-5 - "dry" system at different pressures (kbar): 2 - 5; 3 - 15; 4 - 20; 5 - 25; 6-7 - PH2O=5 and 7.5 kbar respectively; 8 - 5 kbar in the presence of acid fluid (H2O+HCl). Arrows show isobaric temperature increase. Fields: for A - tonalities; B - granodiorites; C - adamelities; D - trondhjemities; E - granites.
References:
Persikov E.S., Bukhtiyarov P.G. Rheology and physico-chemical properties of magmatic melts.
1. The study has been performed on the H2 and H2O solubility in jadeite and albite melts at Pfl to 5 kbar using NMR spectroscopy method to analyse H2 and H2O species in the melts (in collaboration with the scientists of Sapporo University, Japan). The structure of fluid-bearing and "dry" glasses (quenched melts) has been studied in Pfl range to 5 kbar and Plit to 70 kbar. It was found: a) noticable alteration in the structure of an albite melt at H2O dissolution and more negligible effect of high H2 pressures on the structure of an albite melt; b) the presence of a molecular hydrogen in microbubbles of a fluid phase of albite melts at the interaction with hydrogen [Persikov, Bukhtiyarov,1991].
2. We were first to study experimentally the rheological properties (viscosity, activation energy) of water containing diopside melts at PH2O to 5 kbar. The viscosity and activation energy values of water containing jadeite melts at PH2O to 5 kbar were obtained by calculation using a refined model of Persikov which allows to take into account the effect of the ratio Na/Al on the rheological properties of alumosilicate melts including water containing ones. It was shown that viscosity and activation energy of viscous flow of jadeite melts decrease essentially with H20 pressure, i.e. H2O solubility in the melt is followed by its polymerization and increase in basicity as compared with a "dry" melt. By contrast, the viscosity of a diopside melt increases under H2O pressure as compared to that at atmospheric and corresponding lithostatic pressures. This experimentally proves the theoretical forecast [Persikov, 1991] on the basically new mechanism of water dissolution in a depolymerized (metasilicate) diopside melt when H2O dissolution in the melt is accompanied by depolymerization, i.e. increase in its acidity.
3. The viscosities of albite, jadeite, and diopside melts were for the first time studied at 40 kbar and temperatures to 1850oC. It is shown that viscosity of highly polymerized (at P=1 atm) albite and jadeite melts at P=40 kbar, decreases by 2-3 orders of magnitude, whereas the viscosity of the diopside depolymerized (metasilicate) melt, on the contrary, increases by an order of magnitude at the same pressure (Persikov E.S., Bukhtiyarov P.G., 1994). These important results agree well with the theoretical forecast (Persikov,1990) which suggests that high pressure affects the structure of acid melts provoking their depolymerization due to the alterations in coordination surrounding by oxygen of network forming cation (AlIV  AlVI). Metasilicate structure (diopside), by contrast, compacts under pressure hampering the relative migrations of structure layers. This agree well with the behavior of conventional (New-ton's) inorganic liquids.
AlVI). Metasilicate structure (diopside), by contrast, compacts under pressure hampering the relative migrations of structure layers. This agree well with the behavior of conventional (New-ton's) inorganic liquids.
4. A theoretical analysis of pressure dependence of the viscosity of magmatic melts in the pressure range up to 240 kbar was done. It is shown that generalized dependence of the viscosity of magmatic melts on pressure has two minima and, likely, one maximum. The position of the first minimum for a jadeite melt was experimentally established to be 95 kbar which corresponds to the complete
12
transition of Al cations in the jadeite melt from the forth to sixth coordination by oxygen. Pressure corresponding to the first viscosity minimum was shown to be dependent on the melt composition and shifts towards lower pressures with an increase of the melt basicity.
The pressure for an eutectic melt of the diopside-anorthite (Di58An42) system decreases about by half (50 kbar) as compared to jadeite melt (90 kbar) (Persikov E.S. et all, 1991). It is shown that:
- the viscosity temperature dependence for all the compositions of the Di-Ab-H2O system studied is of the exponential character with the constant value of preexponential factor and activation energies of viscous flow do not depend on T for the near liquidus temperature range;
- the viscosity and activation energy dependence on pressure for all the compositions of the system Ab-Di have minima except the compositions Di (Persikov E.S., Bukhtiyarov P.G., 1994);
- the values of the activation energies at minimum pressures do not depend on melt composition and are in the first approximation numerically equal to the activation energy for metasilicate melt of any composition;
- pressure in minimum points regularly decreases with the growth in the melt basicity.
This study laid the foundation for the development of a new chemico-structural model to predict mantle and core magmas viscosity.
References:
Litvin Yu.A. The study of phase equilibria of mantle minerals and primary magmas in the systems Ol-Opx-Cpx-Sp/Gr and Fo-En-Jd up to 165 kbar.
1. Theoretical and experimental investigations of base mantle systems Ol-Opx-Cpx-Sp/Gr which in relation to Na2O, FeO, CaO, MgO, Al2O3, and SiO2 contents correspond to the standard compositions of primitive spinel- and garnet-lherzolite susbstance of the upper mantle (of KLB-1 type) has been completed. In order to develop physico-chemical foundations of mantle magmatism in the framework of Ol-Cpx-Coes system liquidus equilibria of multicomponent mantle substance represented in natural conditions by differentiated peridotite-pyroxenite-eclogite series were generalized.
The additional experiments in the system forsterite-corundum were carried out, and the reaction with formation of pyrope above 30 kbar was established. In the alkaline system Fo-Jd-Di the reaction with formation of pyrope, enstatite and Na-Mg-silicate Na2Mg2Si2O7 above 45 kbar was found. In the eclogitic system omphacite-garnet-corundum-kyanite an invariant eutectic equilibrium was established. The key liquidus equilibria for the Ol-Cpx-Cor-Coes are: invariant peritectics Ol, Opx, Cpx, Gr, L, univariant curve Ol, Cpx, Gr, L as well as divariant field Cpx, Gr, L extended to the peritectic peridotite-pyroxenite subsystem eutectic eclogite. Based on the analysis of the liquidus equilibria of the olivine-clinopyroxene-corundum-coesite system and an account taken of the reaction relations of forsterite and jadeite components a physico-chemical model of fractional crystallization of the primary komatite magma above 45 kbar was proposed (Litvin, in press).
2. Investigation of the melting diagram of the En-Jd system and melting curve of Jd were completed. Experiments were carried out in the State Univerasity of New York at Stony Brook, USA, using a multianvil apparatus of the "split sphere" type. In the 25-165 kbar pressure range the melting curve of jadeite obeys the Simons equation:
P,GPa = 2.5 + 2.01 [(T(oK)/1543) 3.7 - 1]
The temperature of jadeite congruent melting changes with pressure faster than that of any other mantle mineral and at P=130 kbar is higher than melting temperature of enstatite and at P=165 kbar than forsterite. The system En-Jd remains peritectic up to P=80 kbar, the peritectics composition, herewith, becomes more magnesial with pressure and jadeite content in it decreases from 60 to 30 mol.%. At P=80 kbar, the peritectic type of the system changes for the eutectic which is accompanied by the appearence of azeotropic minimum and maximum on the liquidus of solid solutions of jadeite clinopyroxene. The results obtained can be applied to the problem of the differentiation of mantle substance and present an additional argument to the hypothesis of the fractionation crystallization of mantle magmatic melts in the framework of the concept on the "magmatic ocean" (Litvin, Gasparik, 1993,1996).
References:
13
Marakushev A.A. Catastrophic factors in the Earth's evolution. The nature and role of the fluid-magmatic activity.
The model for the earth origin was developed, and the primary catastrophic factors (endogenic and cosmogenic) affecting its normal evolution were defined.
The original (comet) hypothesis of the earth origin was developed, which is based on the conclusion of two-stage earth evolution. The first stage was the primary stratification under the pressure of the thick hydrogen shell, and the second involved the transformation of the earth crust and mantle by the fluid flows emitted by the molten earth core [1].
The study of ring impact structures (e. g. Puchezh-Katun' structure) showed that there are cosmogenic (small bowl-shaped craters) and endogenic structures. The latter are often diamond-bearing and are referred as so-called astroblemes. Their endogenic origin is doubtless now, and the thermodynamic model is developed for the endogenic impactogenesis, which is accompanied by the formation of diamond and other high-pressure minerals [2, 3].
References:
Salova T.P., Stolyarova T.A., and Epel'baum M.B. The relations between aqueous species dissolved in rhyolitic and quartz glasses: calorimetric studies.
Calorimetric analysis of water-bearing silicates was performed (calorimetry of matter dissolution in aggressive media) in order to determine the enthalpies of binding water (molecular or hydroxyl) by silicate glasses and to study the composition effect on the enthalpy values. Among the samples studied there were obsidian glasses with different water content (0.69, 3.86, and 5.37 wt %), zeolite, and quartz glass (2.92 wt % H2O). Zeolite is of interest as close to rhyolitic glass in composition, but containing all water in the molecular form. PMR-spectroscopic studies indicated that water-bearing quartz glass contained water only in OH- form, whereas the water-bearing obsidian glass contained both OH- and molecular H2O. From the enthalpies of dissolution experimentally obtained in the water-glass systems, the enthalpies of mixing were calculated as follows: 0.9-2.1 kcal/mol for obsidian glass (depending on water content), 1.88 kcal/mol for zeolite, and 8.23 kcal/mol for quartz glass. The contributions of OH- and H2O(mol.) to the thermal effect of water dissolution in glass were estimated. The enthalpies of dissolution of molecular water in zeolite and obsidian glasses were determined at 2.54 and 0-4.0 kcal/mol H2O, respectively. The enthalpies of dissolution of OH- in water-bearing obsidian glasses and quartz glass are 7-10 and 28.8 kcal/mol H2O, respectively.
The obtained enthalpies of dissolution of hydroxyl water in glasses of different composition indicate a significant effect of glass composition on the enthalpy of OH-binding reaction. Binding OH- by quartz and aluminosilicate glasses occurs in different manner. The dissolution of water in rhyolitic glass and subsequent OH- formation probably occurs through the interaction of water with the feldspar component of the melt (Salova et al., 1997).
References:
14